Danh mục: NGOẠI NGỮ
-
Avery’s Diseases of the Newborn 11E 2024.pdf
Download Avery’s Diseases of the Newborn 11E 2024.pdf Easily In Format For Free
PrefaceDiseases of the Newborn was one of the first books dedicated to the diagnosis and treatment of problems of the neonate. The 1st edition was published in 1960 by Dr. Alexander Schaffer, a well-known Baltimore pediatrician who first coined the terms neonatology and neonatologist. He described neonatology as an emerging pediatric subspecialty concentrating on the “art and science of diagnosis and treatment of disorders of the newborn infant,” and a neonatologist as a “physician whose primary concern lay in that specialty.” Dr. Schaffer served as sole author for both the 1st and 2nd editions (1966) of the book. Dr. Mary Ellen Avery joined Dr. Schaffer as a co-author for the 3rd edition in 1971. Drs. Avery and Schaffer recognized that their book needed multiple contributors with subspecialty expertise as they developed the 4th edition in 1977, and they became co-editors, rather than co-authors. Dr. Schaffer died in 1981 and Dr. H. William Taeusch joined Dr. Avery in 1984 as co-editor for the 5th edition. Dr. Roberta Ballard joined Drs. Taeusch and Avery for the 6th edition in 1991, then titled, Schaffer & Avery’s Diseases of the Newborn. The 7th edition, edited by Drs. Taeusch and Ballard, was published in 1998, and was entitled Avery’s Diseases of the Newborn, in recognition of Dr. Avery’s diligent work on the book through four editions over 20 years. Dr. Christine Gleason joined Drs. Taeusch and Ballard in 2005 as editors for the 8th edition. In 2009, Drs. Avery, Taeusch, and Ballard retired from editing Avery’s, and became “editors emeriti.” Sadly, Dr. Avery passed away in 2011. Her legacy lives on, however, in the title of this book. Dr. Sherin Devaskar joined Dr. Gleason in 2012 as co-editor for the 9th edition—the first edition with accompanying online content. For the 10th edition, Dr. Sandra “Sunny” Juul teamed with Dr. Gleason as co-editor, marking the first time since the 5th edition that all editors were faculty at the same institution. For this new, 11th edition, Dr. Taylor Sawyer, also on the faculty at Dr. Gleason’s institution, joins as co-editor. This edition marks the fourth that Dr. Gleason has co-edited, making her the longest serving editor since Dr. AveryThe 1st edition of Diseases of the Newborn was used mainly for diagnosis, but also included descriptions of early neonatal therapies that had led to a remarkable decrease in the infant mortality rate in the United States: from 47 deaths per 1000 live births in 1940 to 26 per 1000 in 1960. However, a pivotal year for the fledgling subspecialty of neonatology came in 1963, 3 years after the first publication of Diseases of the Newborn, with the birth of President John F. Kennedy’s son, Patrick Bouvier Kennedy. Patrick was a preterm infant, born at 34-35 weeks’ gestation, and his death at 3 days of age from complications of respiratory distress syndrome accelerated the development of infant ventilators, which, coupled with micro-blood gas analysis and the use of umbilical artery catheterization, led to the development of newborn intensive care in the late 1960sAdvances in neonatal surgery and cardiology, along with ongoing technological innovations, stimulated the development of neonatal intensive care units and regionalization of care for sick newborn infants over the next several decades. These developments were accompanied by an explosion of research that improved our understanding of the pathophysiology and genetic basis of diseases of the newborn. This in turn led to spectacular advances in neonatal diagnosis and therapeutics—particularly in the care of preterm infants. Combined, these advances have resulted in significant reductions in infant mortality worldwide: from 6.45% in 1990 to 2.82% in 2019. Current research efforts are focused on decreasing the unacceptable regional, ethnic, and global disparities in infant mortality, improving neonatal long-term outcomes, advancing neonatal therapeutics, preventing newborn diseases, and finally—teaming with our obstetrical colleagues—preventing prematurity. This edition tries—as all prior editions have—to translate the findings of ongoing research into practical advice for use at the bedside by neonatal caregivers.What’s New and Improved About This Edition?
Perhaps the most significant change to this edition is what was removed rather than what was added. We carefully reviewed the 10th edition’s table of contents, examining each chapter with a keen eye on keeping the book targeted on diseases of the newborn, bringing the content more in line with the original editions. Thus, several chapters that were not specifically disease-focused were archived, while chapters in some sections were subdivided into new chapters focused on disease-specific content. This book continues to be thoroughly (and sometimes painfully) revised and updated by some of the best clinicians and investigators in their fields—several of whom are new contributors. Some chapters required more extensive updates than others. For all chapters, however, we challenged authors to decrease the word count, use boxes, tables, and figures to break up dense text, and to do their best to make the content as disease-focused as appropriate. This resulted in a more concise, readable, and hopefully, clinically helpful text. We are so grateful to our authors for their contributions and hope readers appreciate their workDo We Still Need Textbooks?
With the incredible amount of information immediately available on the internet, what’s the value of a textbook? We believe that textbooks, such as Avery’s Diseases of the Newborn, will always be needed by clinicians striving to provide state-of-the-art neonatal care, by educators working to train the next generation of caregivers, and by investigators diligently advancing neonatal research and scholarship. A textbook’s content is only as good as its contributors. This book, like in previous editions, has awesome contributors. The authors were chosen for their expertise and ability to integrate their knowledge into a comprehensive, readable, and useful chapter. They did this in the hope that their syntheses could, as Ethel Dunham wrote in the foreword to the 1st edition, “spread more widely what is already known … and make it possible to apply these facts.” We are grateful that the online content of this textbook enjoys increasing popularity. However, we still find printed copies of this and other books lying dog-eared, coffee-stained, annotated, and broken-spined in places where neonatal caregivers congregate. With each subsequent edition, the authors of Diseases of the Newborn help fulfill Dr. Schaffer’s vision of clearing the underbrush from the last frontier of medicine in preparation for its eagerly anticipated crops of saved neonatal lives. Textbooks connect us to the past, bring us up to date on the present, and prepare and excite us for the future. We will always need them, in one form or another. To that end, we have challenged ourselves to meet, and hopefully exceed, that need—for our field, for our colleagues, and for the babies entrusted to our care.https://drive.google.com/file/d/1aeyLQlmb39KK9JIw1fHouiB3XFQE21gN/view
-
Download Nelson Essentials of Pediatrics 9th Edition- 2023. Easily In PDF Format For Free
PREFACE
This edition was created in the midst of several pandemics, one caused by a virus and
one focused on advancing social justice to overcome structural and personal racism. The
many challenges highlighted by these pandemics are resulting in acceleration of nec-
essary changes in medical education. These changes must be built on a foundation of
evidence-based knowledge and heightened awareness. Our goal as the editors and authors
of this textbook is not only to provide the classic, foundational knowledge we use every day,
but also to include recent advances in a readable, searchable, and concise text for medical
learners at all levels. Mastering this knowledge, when combined with mindful experiences
in the rapidly changing world of medicine, will allow our readers to develop the practical
wisdom needed to serve our patients and their families.We hope that this text will help you investigate the common and classic pediatric disor-
ders in a time-honored, logical format, helping you to both acquire and apply knowledge
needed to provide high value care. We are honored to be part of the journey of the thou-
sands of learners who rotate through pediatrics, those who will become new providers of
pediatric care in the years to come, and those who continue to build on their knowledge.CARE OF CHILDREN IN SOCIETY
Health care professionals need to appreciate the interactions
between medical conditions and social, economic, and envi-
ronmental influences associated with the provision of pediat-
ric care. New technologies and treatments improve morbidity,
mortality, and the quality of life for children and their families,
but the costs may exacerbate disparities in medical care. The
challenge for pediatricians is to deliver care that is socially equi-
table; integrates psychosocial, cultural, and ethical issues into
practice; and ensures that health care is available to all children.CURRENT CHALLENGES
Challenges that affect children’s health outcomes include access
to health care; health disparities; supporting their social, cogni-
tive, and emotional lives in the context of families and commu-
nities; and addressing environmental factors, especially poverty.
Early experiences and environmental stresses interact with the
genetic predisposition of every child and, ultimately, may lead
to the development of diseases seen in adulthood. Pediatricians
have the unique opportunity to address not only acute and
chronic illnesses but also environmental and toxic stressors to
promote wellness and health maintenance in children.Many scientific advances have an impact on the growing role
of pediatricians. Newer genetic technologies allow the diagnosis
of diseases at the molecular level, aid in the selection of medi-
cations and therapies, and may provide information on prog-
nosis. Prenatal diagnosis and newborn screening improve the
accuracy of early diagnosis and treatment, even when a cure is
impossible. Functional magnetic resonance imaging allows a
greater understanding of psychiatric and neurologic problems.
Challenges persist due to the increasing incidence and prev-
alence of chronic illness. Chronic illness is now the most com-
mon reason for hospital admissions among children (excluding
trauma and newborn admissions).In older children, mental
illness is the main non–childbirth-related reason for hospital-
ization. Pediatricians must also address the increasing concern
about environmental toxins and the prevalence of physical,
emotional, and sexual abuse, and violence. World unrest, ter-
rorism, and a global pandemic have caused an increased level of
anxiety and fear for many families and children.To address these ongoing challenges, many pediatricians
now practice as part of a health care team that includes psy-
chiatrists, psychologists, nurses, and social workers. This
patient-centered medical home model of care is designed to
provide continuous and coordinated care to maximize health
outcomes. Other models, such as school-based health and
retail medical facilities, may improve access but may not sup-
port continuity and coordination of care.Childhood antecedents of adult health conditions, such
as alcoholism, depression, obesity, hypertension, and hyper-
lipidemias, are increasingly recognized. Infants who are rela-
tively underweight at birth due to maternal malnutrition are
at higher risk of developing certain health conditions later
in life, including diabetes, heart disease, hypertension, met-
abolic syndrome, and obesity. Improved neonatal care results
in greater survival of preterm, low birthweight, or very low
birthweight newborns, increasing the number of children with
chronic medical conditions and developmental delays with
their lifelong implications. Childhood exposure to adverse
experiences such as abuse, divorce, and violence increases the
risk of diabetes, cardiovascular disease, and mental health dis-
orders in adults.https://drive.google.com/file/d/1tEM_c4zRCp3zwhI8y-jh6_ON-Yjb8vY9/view
-
Red Book Atlas of Pediatric Infectious Diseases 5th Edition -2023 pdf
Download Red Book Atlas of Pediatric Infectious Diseases 5th Edition -2023 pdf Easily In Format For Free
-
Vancomycin Therapeutic Guidelines: A Summary
IDS A GUIDELINE S
Vancomycin Therapeutic Guidelines: A Summary
of Consensus Recommendations from the Infectious Diseases Society of America, the American Society of Health-System Pharmacists, and the Society
of Infectious Diseases Pharmacists
Michael J. Rybak,1,2,3 Ben M. Lomaestro,4 John C. Rotschafer,5 Robert C. Moellering, Jr.,6,7,8 Willam A. Craig,9 Marianne Billeter,10 Joseph R. Dalovisio,11 and Donald P. Levine3
1Anti-Infective Research Laboratory, Department of Pharmacy Practice, College of Pharmacy & Health Sciences, and 2Department of Medicine, School of Medicine, Wayne State University, and 3Detroit Receiving Hospital & University Health Center, Detroit, Michigan; 4Albany Medical Center, Albany, New York; 5Department of Experimental and Clinical Pharmacology, College of Pharmacy, University of Minnesota, Minneapolis; 6Shields Warren-Mallinckrodt Medical Research, 7Harvard Medical School, and 8Department of Medicine, Beth Israel Deaconess Medical Center,
Boston, Massachusetts; 9University of Wisconsin School of Medicine and Public Health, Madison; and 10Oshsner Medical Centers and 11Department of Infectious Diseases, Oschsner Health System, New Orleans, Louisiana
Practice guidelines for therapeutic monitoring of vancomycin treatment for Staphylococcus aureus infection in adult patients were reviewed by an expert panel of the Infectious Diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. A literature review of existing evidence regarding vancomycin dosing and monitoring of serum concentrations, in addition to patient outcomes combined with expert opinion regarding the drug’s pharmacokinetic, pharmacodynamic, and safety record, resulted in new recommendations for targeting and adjustment of vancomycin therapy.
EXECUTIVE SUMMARY
Adjustment and targeting of specific serum concentra- tions of vancomycin in patients have been the subject of debate for many years. The primary premise for monitoring and adjustment of serum vancomycin con- centrations is based on the perceived need to achieve serum concentrations at some multiple above the min- imum inhibitory concentration (MIC) for the offend- ing organisms and the avoidance of potential adverse effects, such as ototoxicity or nephrotoxicity. The lack
Received 9 April 2009; accepted 10 April 2009; electronically published 1 July
2009.
These guidelines were developed and issued on behalf of the Infectious Diseases Society of America.
Reprints or correspondence: Michael J. Rybak, Anti-Infective Research Laboratory, Wayne State University, 259 Mack Ave., Detroit, MI 48201 ([email protected]).
Clinical Infectious Diseases 2009; 49:325–7
© 2009 by the Infectious Diseases Society of America. All rights reserved. 1058-4838/2009/4903-0001$15.00
DOI: 10.1086/600877
of well-designed randomized clinical evaluations or data to support a clear relationship between specific serum concentrations and patient outcome has been the overriding contributor to this controversy. Unfor- tunately, the controversy has resulted in variable clinical practice methods. In some cases, monitoring is infre- quent or avoided. In other cases, monitoring and dos- age adjustment is overly aggressive.
The relationship between serum concentrations and treatment success or failure in serious Staphylococcus aureus infections has recently been established. Failure rates exceeding 60% for S. aureus displaying a vanco- mycin MIC value of 4 mg/L prompted recommenda- tions in 2006 from the Clinical and Laboratory Stan- dards Institute to lower the breakpoint for susceptibil- ity from 4 to 2 mg/L and in 2008 from the US Food and Drug Administration. Recently, a number of stud- ies have established a relationship between vancomy- cin treatment failures and infections in patients with methicillin-resistant S. aureus displaying an MIC of
Downloaded from http://cid.oxfordjournals.org/ at University of Texas at Austin on December 5, 2014
Vancomycin Therapeutic Guidelines • CID 2009:49 (1 August) • 325
2 mg/L. Vancomycin displays concentration-independent ac- tivity against S. aureus, with the area under the concentration curve (AUC) divided by the MIC as the primary predictive pharmacodynamic parameter for efficacy. On the basis of in vitro, animal, and limited human data, an AUC/MIC value of 400 has been established as the pharmacokinetic-pharmaco- dynamic target. To achieve this target, larger vancomycin doses and high trough serum concentrations are required. Although vancomycin administration is associated with some adverse ef- fects, the committee felt that the potential benefit of increased drug dosage was worth the risk of mostly reversible adverse events.
LITERATURE REVIEW, ANALYSIS, AND CONSENSUS
The expert panel reviewed the literature on pharmacokinetics, pharmacodynamics, efficacy, resistance, and toxicity of van- comycin.[1] A computerized literature search of PubMed for all relevant data published in the English language from 1958 through 2008 was conducted and forms the basis of these rec- ommendations. The quality of the studies was rated, and con- sensus recommendations were graded using the classification scheme of the Canadian Medical Association (table 1). It should be noted that the majority of the published vancomycin-mon- itoring studies were not randomized but consisted of obser- vational data. In addition, data from pediatric studies were not included; therefore, the recommendations are only for adult patients. The committee members were assigned specific topic areas and met via several teleconferences and in person to re- view the draft guidelines. The draft monitoring guidelines were circulated among committee members and were reviewed by each participating professional society for comments and re-
visions. The final guidelines were reviewed and approved by the 3 supporting organizations.
SUMMARY OF RECOMMENDATIONS
Therapeutic Vancomycin Dose Adjustment and Drug Monitoring Dosage. Initial vancomycin dosages should be calculated on the basis of actual body weight, including for obese patients. Subsequent dosage adjustments should be based on actual se- rum concentrations, to achieve targeted therapeutic concentra- tions. Continuous infusion regimens are unlikely to substantially improve patient outcome, compared with intermittent dosing. (Level of evidence, II; grade of recommendation, A.)
Peak versus trough concentrations. Trough serum van- comycin concentrations are the most accurate and practical method of monitoring the effectiveness of vancomycin. Trough serum concentrations should be obtained just before the fourth dose, at steady-state conditions. (Note that steady-state achieve- ment is variable but occurs approximately just before the fourth dose.) (Level of evidence, II; grade of recommendation, B.)
Avoidance of development of resistance. On the basis of the evidence suggesting that S. aureus exposure to trough serum concentrations of !10 mg/L can produce strains with vanco- mycin–intermediately susceptible S. aureus (VISA)–like char- acteristics, it is recommended that trough serum vancomycin concentrations always be maintained at 110 mg/L to avoid the development of resistance. (Level of evidence, III; grade of rec- ommendation, B.)
Recommended trough serum concentrations and dosage adjustments. On the basis of the potential to improve pen- etration, to increase the probability of optimal target serum concentrations, and to improve clinical outcomes of compli- cated infections, such as bacteremia, endocarditis, osteomyelitis,
Downloaded from http://cid.oxfordjournals.org/ at University of Texas at Austin on December 5, 2014
Table 1. Definition of quality of evidence and strength of recommendation.
Assessment Type of evidence
Quality of evidence
Level I Evidence from at least 1 properly designed randomized, controlled trial
Level II
Evidence from at least 1 well-designed clinical trial, with- out randomization; from cohort or case-controlled ana- lytic studies (preferably from 11 center); from multiple time series; or from dramatic results of uncontrolled experiments
Level III Evidence from opinions of respected authorities, based on clinical experience, descriptive studies, or reports of expert committees
Strength of recommendation
Grade A Good evidence to support a recommendation for use
Grade B Moderate evidence to support a recommendation for use
Grade C Poor evidence to support a recommendation
NOTE. Adapted from the Canadian Task Force on the Periodic Health Examination [2].
326 • CID 2009:49 (1 August) • Rybak et al
meningitis, and hospital-acquired pneumonia caused by S. au- reus, trough serum vancomycin concentrations of 15–20 mg/ L are recommended. Trough serum vancomycin concentrations in that range should achieve an AUC/MIC of 1400 for most patients if the MIC is !1 mg/L. (Level of evidence, III; grade of recommendation, B.) To achieve rapid attainment of this target concentration for seriously ill patients, a loading dose of 25–30 mg/kg (based on actual body weight) can be considered. (Level of evidence, III; grade of recommendation, B.) A targeted AUC/MIC of 1400 is not achievable with conventional dosing methods if the vancomycin MIC is “2 mg/L for a patient with normal renal function (i.e., creatinine clearance, 70–100 mL/ min). Therefore, alternative therapies should be considered. Vancomycin dosages of 15–20 mg/kg (based on actual body weight) given every 8–12 h are required for most patients with normal renal function to achieve the suggested trough serum concentrations when the MIC is !1 mg/L. It should be noted that currently available nomograms were not developed to achieve these targeted end points. Individual pharmacokinetic adjustments and verification of achievement of target serum concentrations are recommended. When individual doses ex- ceed 1 g (e.g., 1.5 and 2 g), the infusion period should be extended to 1.5–2 h. (Level of evidence, III; grade of recom- mendation, B.)
Vancomycin toxicity. There are limited data suggesting a
direct causal relationship between toxicity and specific serum vancomycin concentrations. There are also conflicting data characterized by confounding nephrotoxic agents, inconsistent and highly variable definitions of toxicity, and the inability to examine the time sequence of events surrounding changes in renal function secondary to vancomycin exposure. A patient should be considered to have vancomycin-induced nephrotox- icity if multiple (at least 2 or 3 consecutive) high serum cre- atinine concentrations (increase of 0.5 mg/dL or 150% increase from baseline, whichever is greater) are documented after sev- eral days of vancomycin therapy in the absence of an alterna- tive explanation. (Level of evidence, II; grade of recommen- dation, B.)
Monitoring of serum concentrations to reduce toxicity.
Available evidence does not support monitoring of peak serum vancomycin concentrations to decrease the frequency of ne- phrotoxicity. (Level of evidence, I; grade of recommendation, A.) Monitoring of trough serum vancomycin concentrations to reduce nephrotoxicity is best suited for patients receiving aggressive dose targeting to produce sustained trough serum concentrations of 15–20 mg/L or who are at risk of toxicity, such as patients receiving concurrent treatment with nephro- toxins. (Level of evidence, III; grade of recommendation, B.) Monitoring is also recommended for patients with unstable renal function (either deteriorating or significantly improving
function) and for patients receiving prolonged courses of ther- apy (13–5 days). (Level of evidence, II; grade of recommen- dation, B.) All patients receiving prolonged courses of vanco- mycin treatment should have at least 1 steady-state trough serum concentration measured just before the fourth dose. Fre- quent monitoring (11 measurement of trough concentration before the fourth dose) for short-course therapy (!5 days) or for lower-intensity dosing (targeted to attain trough serum van- comycin concentrations of !15 mg/L) is not recommended. (Level of evidence, II; grade of recommendation, B.) There are limited data to support the safety of sustained trough serum vancomycin concentrations of 15–20 mg/L. When this target range is desired, once-weekly measurements of trough concen- trations for hemodynamically stable patients is recommended. Frequent (in some instances, daily) monitoring of trough con- centrations is advisable to prevent toxicity in hemodynamically unstable patients. The exact frequency of monitoring is often a matter of clinical judgment. (Level of evidence, III; grade of recommendation, B.) Data on comparative vancomycin toxicity for continuous versus intermittent administration are conflict- ing, and no recommendation can be made. Monitoring of se- rum vancomycin concentrations to prevent ototoxicity is not recommended, because this toxicity is rarely associated with monotherapy and does not correlate with serum vancomycin concentrations. Monitoring may be more important when other ototoxic agents, such as aminoglycosides, are adminis- tered. (Level of evidence, III; grade of recommendation, B.)
Acknowledgments
Potential conflicts of interest. M.J.R. received research grants from Astellas, Cubist, Forest, and Pfizer; consulted for Astellas, Cubist, Forest, Ortho-McNeil, and Targanta; and has served on speakers’ bureaus for Cubist, Wyeth, Pfizer, and Targanta. J.C.R. has received research grants from Ortho-McNeil and Astra-Zeneca; has been a consultant for Ortho- McNeil, Schering, Cubist, Wyeth, Pfizer, Theravance, Optimer, and Bayer; and has served on speakers’ bureaus for Ortho-McNeil, Schering, Pfizer, Wyeth, and Cubist. R.C.M. was a former consultant for Eli Lilly. W.A.C. has received research funding from Johnson & Johnson and Astra-Zeneca and has been a consultant for Bristol-Myers Squibb and Forest Pharma- ceuticals. M.B. has received research funding from Cubist and has served on speakers’ bureaus for Sanofi-Pasteur, Pfizer, and Ortho-McNeil. J.R.D. has received a consulting fee to testify on Capitol Hill regarding the clinical impact of the methicillin-resistant S. aureus outbreak in the United States; no product-specific data were discussed. B.M.L. and D.P.L.: no conflicts.
References
- Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 2009; 66:82–98. Available at: http://www.ashp.org/ DocLibrary/BestPractices/BPVancoAJHP.aspx. Accessed 25 June 2009.
- Canadian Task Force on the Periodic Health Examination. The periodic health examination. Can Med Assoc J 1979; 121:1193–254.
Downloaded from http://cid.oxfordjournals.org/ at University of Texas at Austin on December 5, 2014
Vancomycin Therapeutic Guidelines • CID 2009:49 (1 August) • 327
-
VANCOMYCIN INFUSION REACTION
 Official reprint from UpToDate® www.uptodate.com
Official reprint from UpToDate® www.uptodate.com©2023 UpToDate®
Vancomycin hypersensitivity
Author: Peter F Weller, MD, MACP
Section Editor: N Franklin Adkinson, Jr, MD
Deputy Editor: Anna M Feldweg, MD Contributor Disclosures
All topics are updated as new evidence becomes available and our peer review process is complete.
Literature review current through: Jul 2023. | This topic last updated: Apr 28, 2023.
INTRODUCTION
Vancomycin causes several different types of hypersensitivity reactions, ranging from localized skin reactions to generalized cardiovascular collapse. The most common adverse reaction is vancomycin infusion reaction (VIR). VIR is a rate-dependent infusion reaction, not a true allergic reaction.
Vancomycin hypersensitivity will be reviewed here. Other antibiotics that commonly cause hypersensitivity reactions include the beta-lactam antibiotics and sulfonamides, and hypersensitivity reaction to these drugs are discussed separately. (See “Penicillin allergy: Immediate reactions” and “Sulfonamide allergy in HIV-uninfected patients”.)
TERMINOLOGY
The terms “red man syndrome” or “red neck syndrome” have commonly been used to describe vancomycin infusion reaction (VIR). Another term that is variably used is vancomycin flushing syndrome or reactions. This terminology originated from the dramatic erythema that develops in some patients in response to infusion of vancomycin, but the terms could be misconstrued as insulting to specific groups of people in the United States, and the emphasis on flushing, which is a prominent feature but not the only one, may lead to the reaction being missed in patients with darker skin [1,2]. Thus, the term “vancomycin infusion reaction” is preferred.
VANCOMYCIN INFUSION REACTION
The most common adverse reaction to vancomycin is “vancomycin infusion reaction (VIR),” previously known as “red man syndrome,” although we will avoid further use of this term for the reasons outlined previously. (See ‘Terminology’ above.)
Route of administration — VIR occurs principally with parenteral administration of vancomycin. Rarely, VIR has been caused by topical administration of vancomycin powder [3]. In contrast, oral administration of vancomycin in subjects with Clostridioides difficile infections does not usually result in systemic absorption [4]. However, for some patients, especially those with impaired kidney function or other abnormalities, oral administration can lead to detectable serum levels of the medication, and VIR to oral vancomycin may be possible [5].
Signs and symptoms — VIR may develop with the first administration of vancomycin. It is characterized by flushing, erythema, and pruritus, usually affecting the upper body, neck, and face more than the lower body. Pains and muscle spasms in the back and chest, dyspnea, and hypotension may also occur [6,7]. Otherwise unexplained hypotension has been reported [8,9]. In a retrospective review of multiinstitutional electronic medical records, amongst 3165 recipients of vancomycin, 491 experienced adverse drug reactions, of which 134 were immediate hypersensitivity reactions [10].
VIR is rarely life threatening, although severe cardiovascular toxicity and even cardiac arrest can occur [8,11]. Immunoglobulin E (IgE) mediated anaphylaxis can present with symptoms similar or identical to those of severe VIR, and clinicians should be mindful of this alternative diagnosis. Unlike VIR, an IgE-mediated reaction to vancomycin does not occur with initial administration but instead requires sensitization to develop from a previous course of vancomycin. (See ‘IgE-mediated anaphylaxis’ below.)
Mechanism — VIR is an idiopathic reaction, which is not thought to involve drug-specific antibodies. VIR is a form of pseudoallergic drug reaction, which is an adverse drug reaction with signs and symptoms that mimic immunologic drug allergies but in which IgE- mediated immunologic mechanisms have not been demonstrated.
Studies in animals indicate that vancomycin directly activates mast cells, resulting in release of vasoactive mediators, such as histamine [12,13]. A mast cell specific G-protein
coupled receptor in mice and its human orthologue, MRGPRX2, have been identified as mediating pseudoallergic mast cell activation, as elicited by candidate agents, including ciprofloxacin [14]. In this study, vancomycin was not evaluated, although vancomycin was shown to activate human mast cells through MRGPRX2 in an in vitro study [15]. (See “Mast cells: Surface receptors and signal transduction”, section on ‘MRGPRX2’.)
In several human studies, the degree of elevation in serum histamine was related to the clinical severity of VIR [16-18]. However, in other studies, increased serum histamine concentrations did not predict VIR, and VIR occurred without detectable elevations in plasma histamine [19], suggesting either that other mediators may be involved or that plasma histamine is not a sufficiently sensitive marker for mast cell activation localized to the skin.
Relationship to infusion rate — VIR is usually a rate-related infusion reaction, as illustrated by the following observations [16,20-22]:
- In one study, 10 healthy presurgical patients received rapid infusions of 1 gram of vancomycin over 10 minutes [20]. All developed VIR, seven had severe cutaneous reactions, and five had a reduction in blood pressure of 20 percent or more, necessitating discontinuation of the infusion.
- In a report of 10 adult male volunteers, the incidence and severity of VIR following the infusion of 1 gram of vancomycin over either one or two hours was compared [16]. Eight subjects developed VIR (two severe, three moderate, and three mild) with the one-hour infusion compared with three (all mild) with the two-hour infusion. However, two studies with a total of 62 hospitalized patients with serious infections found a much lower risk of VIR (less than 10 percent) following 1 gram infused over one hour [17,19].
Predisposing medications — Mast cells are more easily activated when vancomycin is given in combination with certain other medications. The combination of vancomycin and opioids (eg, morphine, meperidine, codeine) enhances dose- or rate-related mast cell degranulation [23]. Adverse reactions can occur following administration of vancomycin in patients being treated with an opioid or following the administration of opioids in patients being treated with vancomycin [24].
Similar interactions can occur between vancomycin and radiocontrast dye, some muscle
relaxants used in general anesthesia, and any other agents that potentiate mast cell degranulation ( table 1). Thus, when possible, these agents should not be administered simultaneously or in close approximation with vancomycin.
Prevention of initial reactions — Prevention of VIR involves the use of slower infusion rates and, in some situations, premedication.
Slower infusion rates — To avoid VIR, vancomycin should be infused at a rate no higher than 10 mg/minute or, for a 1 gram dose, over a minimum of 100 minutes (whichever results in a slower infusion) [22]. We advise even slower rates of infusion for patients who are also receiving opioids or other medications that predispose to mast cell activation
( table 1). (See ‘Predisposing medications’ above.)
Premedication — Empiric premedication to prevent VIR is not usually necessary for patients who are receiving vancomycin for the first time at rates of infusion ≤10 mg/min. We generally do not administer premedication for doses ≤500 mg given over one hour or doses of 500 mg to 1 gram given over two hours.
In contrast, empiric premedication with antihistamines is commonly employed if more rapid infusions of vancomycin are required in emergency or presurgical settings. We administer premedication for patients receiving vancomycin at high rates of infusion (more than 10 mg/minute or 1 gram over one hour). Oral antihistamines are preferred when possible. Although H1 antihistamines may be sufficient for mildly increased infusion rates, we suggest administration of both an H1 and H2 antihistamine to minimize the likelihood of a reaction if significantly faster rates are used (eg, 1 gram over 10 minutes).
The efficacy of pretreatment with antihistamines in reducing the incidence and severity of VIR was evaluated in the following studies:
- A randomized trial of 33 patients found that pretreatment with diphenhydramine (50 mg orally) completely prevented VIR in a group of patients receiving 1 gram of vancomycin over 60 minutes [17]. Reactions occurred in 47 percent of the placebo group compared with none in the diphenhydramine group.
- In a randomized trial of very rapid infusions (1 gram over 10 minutes) in 30 presurgical patients, oral premedication with both H1 and H2 antihistamines was given [20]. Subjects received oral diphenhydramine (≤1 mg per kg) plus oral
cimetidine (≤4 mg per kg) one hour before infusion. The incidence and severity of VIR were significantly lower in the antihistamine-treated group, although one antihistamine-treated patient had intolerable itching and could not complete the infusion. Hypotension did not occur in the antihistamine group but developed in 50 percent of patients in the placebo group.
- The same investigators performed another randomized trial in 40 patients using the same medications, doses, and setting as in the previous trial (ie, 1 gram over 10 minutes), although the antihistamine premedications (diphenhydramine and cimetidine) were administered intravenously [25]. Patients treated with an antihistamine had significantly lower rates of hypotension (11 versus 63 percent) and cutaneous findings (63 versus 100 percent). Itching was severe enough in two antihistamine-treated patients to necessitate discontinuation of the infusion.
Based upon these limited data, premedication with H1 alone may be sufficient to prevent VIR following mildly increased rates of infusion, although even the combination of H1 and H2 antihistamines did not completely prevent VIR following very rapid infusions (1 gram over 10 minutes) of vancomycin. Oral and intravenous antihistamine premedications appear to be similarly efficacious.
Management — The optimal management of VIR has not been evaluated in randomized trials. The approach outlined herein is based upon the author’s clinical experience.
- For mild reactions (eg, flushing that is not bothersome to the patient), symptoms typically resolve in minutes, and antihistamines are usually not necessary. We usually restart the infusion at one-half of the previous rate.
- For moderate reactions (eg, the patient is uncomfortable due to flushing or pruritus but hemodynamically stable and not experiencing chest pain or muscle spasm), we typically interrupt the infusion and treat with diphenhydramine (50 mg orally or intravenously) and famotidine (20 mg intravenously). Symptoms usually subside promptly. The infusion can then be restarted at one-half the original rate or 10 mg/minute, whichever is slower.
- For severe reactions (eg, involving muscle spasm, chest pain, or hypotension), we stop the infusion and treat with diphenhydramine (50 mg intravenously) as well as famotidine (20 mg intravenously) and, if hypotension is present, intravenous fluids.
Once symptoms have resolved, the infusion can be restarted and given over four or more hours. For administration of future doses, we suggest repeat premedication with antihistamines before each dose and infusion over four hours, as well as continuous hemodynamic monitoring during infusions.
- It may be difficult or impossible to distinguish severe VIR from anaphylaxis. Flushing and hypotension are features of both reactions. Hives, laryngeal edema, and wheezing are suggestive of anaphylaxis, and patients with these signs and symptoms should be treated with intramuscular epinephrine, in addition to the measures above. Overviews of the treatment of anaphylaxis in adults and in children (with specific medication doses) are provided in the tables ( table 2 and table 3). Infusions must not be restarted if anaphylaxis is suspected, because slowing the rate and administering premedications will not prevent IgE-mediated anaphylaxis. (See ‘IgE- mediated anaphylaxis’ below.)
Following VIR of any severity, the patient’s medication list should be reviewed to determine if other predisposing medications (eg, opioids) ( table 1) can be identified and discontinued before restarting the infusion.
Recurrent reactions — Some individuals experience recurrent and persistent symptoms despite premedication and slower infusion rates [17,24,26]. These individuals may have mast cells and/or basophils that are easily activated. Patients with mast cell disorders are particularly prone to VIR with vancomycin. (See “Mastocytosis (cutaneous and systemic) in children: Epidemiology, clinical manifestations, evaluation, and diagnosis”, section on ‘Triggers for mediator release’ and “Mastocytosis (cutaneous and systemic) in adults: Epidemiology, pathogenesis, clinical manifestations, and diagnosis”.)
Desensitization can be attempted if there is no equally effective alternative antibiotic and vancomycin is absolutely required. (See ‘Desensitization’ below.)
IgE-MEDIATED ANAPHYLAXIS
Anaphylaxis is an immunologically mediated reaction involving drug-specific IgE antibodies. Anaphylaxis in response to vancomycin administration is believed to be rare, although reactions involving angioedema, respiratory distress, and bronchospasm with demonstrable drug-specific IgE have been described [26-28]. (See “Anaphylaxis: Emergency
treatment”.)
Patients with anaphylactic reactions to vancomycin often have a history of multiple prior exposures. Anaphylaxis does not occur on the first administration of the medication, because prior exposure to the drug is necessary to form drug-specific IgE antibodies.
Clinical manifestations — The symptoms of anaphylaxis include (but are not limited to) urticaria, angioedema, generalized pruritus, tachycardia, hypotension, nausea and vomiting, lightheadedness, and hypotension ( table 4).
Although severe vancomycin infusion reactions (VIR) and anaphylaxis can present with similar signs and symptoms, wheezing and respiratory distress are more common in anaphylaxis, whereas VIR more often involves chest pains causing a sensation of chest tightness. Angioedema is usually seen in anaphylaxis only. However, it may not be possible to distinguish anaphylaxis from severe VIR based upon clinical presentation. The patient should be assumed to have anaphylaxis in such cases and managed accordingly.
Acute management — If anaphylaxis is suspected, the vancomycin infusion should be stopped immediately, and the patient should be treated with intramuscular epinephrine. Overviews of the treatment of anaphylaxis in adults and in children (with specific medication doses) are provided in the tables ( table 2 and table 3). A more detailed discussion of the treatment of anaphylaxis is presented separately. (See “Anaphylaxis: Emergency treatment”.)
Diagnosis — Differentiating between anaphylaxis and severe VIR is usually based upon clinical signs and symptoms. Unfortunately, serum and skin tests cannot reliably discriminate between these two reactions. In addition, vancomycin skin testing has not been validated, and the positive and negative predictive values are unknown.
Serum tests — Serum tryptase levels have been studied in severe reactions to vancomycin [29-35]. Tryptase is stored preformed within mast cell granules and released during mast cell degranulation. Elevations in these mast cell-derived mediators are variably found in IgE-mediated anaphylaxis, although normal levels do not exclude anaphylaxis. (See “Laboratory tests to support the clinical diagnosis of anaphylaxis”, section on ‘Tryptase’.)
Skin testing — Skin testing with vancomycin has not been validated, and the positive and
negative predictive value of the results are not known. However, isolated case reports described reactions that were highly suggestive of IgE-mediated drug allergy in which skin test results were positive [36]. As an example, one patient developed generalized urticaria and respiratory distress after several doses of vancomycin [27]. Intradermal skin tests were positive at 0.1 mcg/mL, whereas control subjects had positive results only at much higher concentrations (>10 mcg/mL). The patient was desensitized over 13 days, after which repeat skin testing was negative, which is a clinical marker of successful desensitization in IgE-mediated reactions. Another case report also documented positive skin tests results that converted to negative following desensitization [37]. (See ‘Desensitization’ below.)
These reports suggest that skin testing with appropriate vancomycin concentrations may reflect clinical reactivity and provide supportive evidence for the clinical diagnosis of anaphylaxis. A positive skin test at concentrations of 1 mcg/mL or lower is strongly suggestive of drug allergy in a patient with a reaction that had features of allergy.
Use of alternate medications — Other antimicrobial agents should be considered for patients who have experienced very severe symptoms in response to vancomycin. Some patients receive vancomycin because of a reported history of allergy to penicillins, yet penicillin may be a superior antibiotic for certain infections, such as native valve endocarditis due to methicillin-sensitive Staphylococcus aureus. (See “Antimicrobial therapy of left-sided native valve endocarditis”.)
Patients are sometimes labeled as penicillin allergic based on a vague past history or may have lost the allergy over time. Presurgical evaluation by an allergy specialist to confirm or exclude penicillin allergy should be arranged whenever possible. Several studies have demonstrated the value of avoiding vancomycin for surgical prophylaxis in patients with a self-reported history of penicillin allergy [38-40]. (See “Penicillin allergy: Immediate reactions”, section on ‘Impact of penicillin allergy on care’.)
There are limited antibiotic options for certain infections, however, such as methicillin- resistant S. aureus, coagulase-negative staphylococci, and ampicillin-resistant enterococci. Daptomycin has been used successfully in a patient intolerant to vancomycin [41].
Alternative agents are discussed in detail in specific topic reviews. (See “Methicillin- resistant Staphylococcus aureus (MRSA) in adults: Treatment of bacteremia” and “Infection due to coagulase-negative staphylococci: Treatment” and “Treatment of enterococcal infections”.)
Glycopeptide antibiotics structurally similar to vancomycin include telavancin, dalbavancin, and oritavancin [42]. (See ‘Use of related drugs’ below.)
Desensitization — Desensitization is a procedure that alters the immune activation by the drug and results in temporary tolerance, allowing the patient with a drug hypersensitivity reaction to receive an uninterrupted course of the medication safely. (See “Rapid drug desensitization for immediate hypersensitivity reactions”.)
Indications — Readministration of vancomycin to a patient with a past severe VIR or possible anaphylaxis may need to be considered when no other antimicrobial of equivalent efficacy is available. Vancomycin desensitization is appropriate for both suspected IgE-mediated reactions (preferably confirmed by skin testing) and may be clinically useful for severe VIR that was refractory to the measures outlined above [24,27,37,43,44]. (See ‘Management’ above.)
Precautions — Desensitization, or any form of reexposure, is contraindicated in patients with the following types of past reactions:
- Exfoliative skin reactions – Reactions involving blistering, peeling, or sloughing of the skin, such as Stevens-Johnson syndrome and toxic epidermal necrolysis (see “Stevens-
Johnson syndrome and toxic epidermal necrolysis: Management, prognosis, and long-term sequelae”)
- Drug reaction with eosinophilia and systemic symptoms (DRESS), also called the drug- induced hypersensitivity syndrome [45,46] (see “Drug hypersensitivity: Classification and clinical features”)
Desensitization is generally not performed in patients with past drug fever, hematologic or renal hypersensitivity reactions, phlebitis, or linear immunoglobulin A (IgA) bullous dermatosis. (See ‘Other forms of hypersensitivity’ below.)
Referral — Consultation with an allergy specialist experienced in adverse drug reactions is recommended if desensitization is under consideration. Precautions regarding desensitization include the following:
- Desensitization is performed immediately before required treatment because maintaining tolerance requires continual exposure to the drug.
- Other concurrent health issues should be as well controlled as possible, particularly cardiopulmonary conditions (eg, heart failure, asthma).
- Patients should optimally not be taking medications that may increase the likelihood of anaphylaxis or interfere with treatment of anaphylaxis, such as angiotensin- converting enzyme inhibitors or beta blockers.
- Desensitizations should be performed in an appropriate medical setting, with proper monitoring and immediate availability of rescue medications and equipment. Desensitizations for IgE-mediated sensitivities to intravenous medications are usually performed in an intensive care unit.
- Documentation of informed consent, including a thorough discussion of risks and benefits of the procedure, is essential.
The safety of desensitization as a general technique is reviewed separately. (See “Rapid drug desensitization for immediate hypersensitivity reactions”, section on ‘Safety’.)
Protocols — A variety of intravenous protocols have been published. Most can be completed over several hours, which is important for patients who are infected and acutely in need of treatment [24,43,47-49]. Other protocols involve intermittent doses that increase incrementally over periods ranging from 2 to 13 days [27,37]. Studies comparing the success rates of different protocols have not been performed.
A protocol that can be completed in several hours is provided ( table 5) [47].
Symptoms during desensitization — Symptoms during desensitization have been noted in as many as 30 percent of cases and are usually mild (eg, flushing, pruritus, limited urticaria). Most symptoms can be managed without discontinuation of the desensitization protocol. Mild symptoms are managed by halting the infusion and treating the symptoms that do not subside spontaneously. Once symptoms have subsided, the last tolerated step is repeated. This “stepping back” may be performed again if needed, or an intermediate step can be inserted after the last tolerated step and before the problematic step by reducing the infusion rate of the problematic step.
If moderate or severe symptoms develop, the infusion should be halted and the symptoms treated. The decision to proceed with desensitization depends upon the patient’s status and need for vancomycin.
There is one report of a patient who failed a rapid protocol and was subsequently successfully desensitized using a 13-day procedure [37].
Duration of effect — Desensitization induces a temporary state of tolerance, allowing the drug to be administered safely as long as the patient remains continually exposed to it.
Once the initial desensitization is complete, the patient can receive subsequent doses normally, and no symptoms are anticipated. However, serum levels of vancomycin must be monitored carefully to ensure that the concentration in the blood does not drop below detectable levels [28,50]. It is not known what threshold level of drug is required to maintain the tolerized state. However, the drug levels should be kept within the therapeutic range if possible, both to maintain tolerance and treat the infection. This may require continuous, rather than intermittent, dose infusions.
If the drug level becomes undetectable, then desensitization should be repeated in order to reintroduce the medication safely. Once the course of treatment is completed, it must be clearly explained to patients that they still have an allergy to vancomycin and would have to be desensitized again if it were required in the future.
OTHER FORMS OF HYPERSENSITIVITY
The most common hypersensitivity reaction to vancomycin is skin rash. Other reactions, not all of which are immune mediated, include hematologic and kidney disorders, drug fever, and phlebitis [51,52].
DRESS — Vancomycin has elicited drug reaction with eosinophilia and systemic symptoms (DRESS), which is also called drug-induced hypersensitivity syndrome [45,53-55]. This drug reaction involves rash, atypical lymphocytosis, frequent but not uniform eosinophilia, and, often, lymphadenopathy. There may be hepatic, kidney, and/or pulmonary involvement.
Treatment involves discontinuing the causative drug and, conventionally, administration of glucocorticoids [46,54]. Amongst an extensive review of electronic medical records of patients developing DRESS, antibiotics accounted for 74 percent, with vancomycin the most common antibiotic (39 percent) overall [56]. (See “Drug reaction with eosinophilia and systemic symptoms (DRESS)”.)
Expression of the HLA-A*32:01 allele was found in 83 percent of 23 cases of vancomycin- elicited DRESS versus 0 percent in vancomycin-tolerant control subjects [57]. The authors
suggest that, while administration of vancomycin often needs to be initiated rapidly, since DRESS will not develop usually until after two weeks of drug administration, testing for the HLA-A*32:01 allele be performed soon after initiation of the drug to assess genetic risks for DRESS development. Any drug culprit in DRESS should be avoided in the future as rechallenge can precipitate severe and fatal reactions.
Dermatologic — The most common form of vancomycin hypersensitivity reaction is skin rash. Other dermatologic reactions include linear IgA bullous dermatosis (LABD) and various other rare disorders.
Maculopapular and urticarial eruptions — Maculopapular skin eruptions are the most frequent dermatologic manifestations of vancomycin hypersensitivity [58-62]. In a systematic review of patients receiving teicoplanin or vancomycin, skin rashes occurred in 57 of 889 (6 percent), while vancomycin infusion reaction (VIR) occurred in 18 of 414 (4 percent) [62]. Urticarial eruptions are also reported [60].
Vancomycin-related linear IgA bullous dermatosis — LABD is an autoantibody- mediated skin reaction to vancomycin (
 picture 1) [63-69]. In a review of immune- mediated reactions to vancomycin, LABD was the most commonly identified immune- mediated adverse reaction [36]. This entity may be confused with toxic epidermal necrolysis, although LABD does not usually involve mucosal membranes [70-72]. In addition, a nonbullous, morbilliform variant of vancomycin-induced LABD has been reported [73]. LABD can appear from one day to one month from the time of initial vancomycin administration. The reaction appears to be idiosyncratic and unrelated to peak or trough serum vancomycin levels. LABD is discussed in more detail separately. (See “Linear IgA bullous dermatosis”.)
picture 1) [63-69]. In a review of immune- mediated reactions to vancomycin, LABD was the most commonly identified immune- mediated adverse reaction [36]. This entity may be confused with toxic epidermal necrolysis, although LABD does not usually involve mucosal membranes [70-72]. In addition, a nonbullous, morbilliform variant of vancomycin-induced LABD has been reported [73]. LABD can appear from one day to one month from the time of initial vancomycin administration. The reaction appears to be idiosyncratic and unrelated to peak or trough serum vancomycin levels. LABD is discussed in more detail separately. (See “Linear IgA bullous dermatosis”.)Rare severe cutaneous reactions — Stevens-Johnson syndrome [74,75], exfoliative dermatitis [76], toxic epidermal necrolysis [77], extensive fixed drug eruption [78], and leukocytoclastic vasculitis [79] have all been described in association with vancomycin use in case reports. Early recognition and discontinuation of the drug are critical.
Desensitization has no efficacy in these reactions and should not be performed for the purposes of circumventing recurrence, as reexposure to the drug could result in a more severe or fatal recurrence of the reaction. (See “Stevens-Johnson syndrome and toxic epidermal necrolysis: Pathogenesis, clinical manifestations, and diagnosis”.)
Hematologic — Hematologic manifestations of vancomycin-related reactions include
leukocytosis, eosinophilia, neutropenia, and immune thrombocytopenia [59,80-85]. Neutropenia tends to occur with longer courses of therapy, and weekly monitoring of the white blood cell count and differential leukocyte counts during prolonged administration is indicated [86]. A case of agranulocytosis in a patient with kidney insufficiency was also reported [18]. Vancomycin should be discontinued if these conditions develop.
Drug-induced fever — Uncommonly, vancomycin has been implicated as a cause of drug- induced fever [51,52,87]. In some instances, fever has occurred concomitant with vancomycin-elicited neutropenia [58,81].
Kidney — Vancomycin may cause nephrotoxicity, especially in patients receiving confounding nephrotoxins or who have kidney insufficiency or altered hemodynamics [88- 90]. (See “Vancomycin: Parenteral dosing, monitoring, and adverse effects in adults”.)
On occasion, vancomycin can also elicit immunologically mediated kidney damage due to acute interstitial nephritis [91-93]. Vancomycin should be discontinued if it is a likely cause of acute interstitial nephritis. (See “Clinical manifestations and diagnosis of acute interstitial nephritis”.)
USE OF RELATED DRUGS
Glycopeptide antibiotic analogs — The agents that share structural similarities with vancomycin include teicoplanin, dalbavancin, oritavancin, and telavancin [42]. Teicoplanin has been in use longer than the other agents, so there is more experience with the adverse reactions it can cause.
Teicoplanin — Teicoplanin, which is not available in the United States, is a glycopeptide antimicrobial with structural similarity to vancomycin, equivalent efficacy in treating invasive beta-lactam-resistant, gram-positive infections, but with apparently lower rates of adverse events, particularly nephrotoxicity and vancomycin infusion reaction (VIR) [62,94- 96].
- Teicoplanin can cause flushing and pruritus, but clinically significant infusion reactions are rare with teicoplanin [94,97,98].
- Teicoplanin has been implicated in perioperative anaphylaxis [99-101].
There is only limited information about the safety of teicoplanin in patients with a previous hypersensitivity reaction to vancomycin:
- Recurrence of vasculitic rash was described in two patients treated with teicoplanin who had previously reacted to vancomycin [102].
- A retrospective series evaluated 117 patients who had drug-induced fever (24 patients), rash (77 patients), both (8 patients), or neutropenia (8 patients) while receiving vancomycin and were switched to teicoplanin [58]. Clinical information and the development of drug-induced fever, rash, or neutropenia with teicoplanin were determined by medical record review. Ten percent of patients developed fever, rash, or neutropenia in response to teicoplanin; there were no fatalities due to drug adverse reactions to teicoplanin. Of note, 50 percent of patients with neutropenia in response to vancomycin also developed neutropenia in response to teicoplanin. Thus, the majority of patients in the series tolerated teicoplanin, with the exception of those with neutropenia, in whom one-half had recurrence.
- Teicoplanin can induce drug reaction with eosinophilia and systemic symptoms (DRESS)/drug-induced hypersensitivity syndrome, like vancomycin [45,53]. Cross- sensitivity to both drugs is possible, as described in at least one case report [45].
Dalbavancin, oritavancin, and telavancin — These analogs of vancomycin are approved for use in the United States, including for skin and skin structure infections [42]. Telavancin is administered daily, while oritavancin and dalbavancin are long lived and are usually given as a single dose. While the long-lasting antimicrobial effects of these agents are beneficial, adverse reactions to these agents may produce persistent symptoms that extend well beyond discontinuation of the drug.
Reports of adverse effects from these agents are limited but appear to be less common than with vancomycin [103].
- VIR-like reactions have been reported with both dalbavancin and oritavancin and can be managed with slower infusion rates [104].
- Anaphylaxis has also been reported with both agents, but details that might distinguish IgE-mediated anaphylaxis from nonimmune-mediated pseudoallergic reactions are not available [104].
Reports of adverse reactions to dalbavancin and oritavancin in patients with previous serious adverse reactions to vancomycin are also limited, and we would advise exploring other options before using related glycopeptide agents. Specifically, alternative antibiotics should be utilized when possible. If the concern is about a VIR-like infusion-related event, appropriate precautions should be taken (slower infusion rate, consideration of relevant coadministered medications) ( table 1).
Graded challenge has be performed in a controlled setting to ascertain if a patient with a previous immediate hypersensitivity reaction to vancomycin could tolerate one of the newer glycopeptide agents. One case report described a graded challenge of dalbavancin in a patient with a past severe hypersensitivity reaction to vancomycin [105]. However, the patient was also receiving opioids and had other conditions that could have potentiated the reaction to vancomycin, so the safety of this approach in other patients with severe hypersensitivity is unclear.
SOCIETY GUIDELINE LINKS
Links to society and government-sponsored guidelines from selected countries and regions around the world are provided separately. (See “Society guideline links: Drug allergy and hypersensitivity”.)
SUMMARY AND RECOMMENDATIONS
- Terminology and clinical manifestations – Vancomycin infusion reaction (VIR; also known as “red man syndrome,” though we avoid the use of this term going forward) is a common adverse reaction to vancomycin. VIR is characterized by flushing, erythema, and pruritus, usually of the upper body. Chest or back pains and hypotension may also occur. It is a rate-related infusion reaction caused by direct activation of mast cells by the drug. Other agents that activate mast cells, such as opioids, muscle relaxants, and radiocontrast media, can predispose patients to developing VIR with vancomycin infusion. (See ‘Vancomycin infusion reaction’ above.)
- Slower infusion rates can prevent VIR – VIR can usually be prevented by administering the drug at rates ≤10 mg/minute (or 1 gram over more than 100 minutes). We do not empirically premedicate patients who have no history of
previous VIR or have never received vancomycin before if the drug is to be administered at these rates. (See ‘Prevention of initial reactions’ above.)
- Premedication for patients who require faster infusions – In patients who require more rapid infusions of vancomycin (ie, at rates exceeding 10 mg/minute or 1 gram over one hour), we recommend antihistamine premedication with at least an H1 antihistamine (Grade 1B). We suggest a combination of H1 and H2 antihistamines (Grade 2C). We administer the combination of diphenhydramine (50 mg orally) and famotidine (20 mg orally) one hour before infusion, although the optimal regimen has not been determined. (See ‘Prevention of initial reactions’ above.)
- Treatment of VIR – VIR is treated by stopping the infusion. Further treatment depends upon the severity of the reaction. The patient’s medication list should be reviewed carefully to determine if other predisposing medications can be identified and discontinued ( table 1). (See ‘Management’ above.)
- For mild reactions (eg, flushing that is not bothersome to the patient), symptoms typically resolve in minutes, and antihistamines are usually not necessary. We usually restart the infusion at one-half of the previous rate.
- For moderate reactions (eg, the patient is uncomfortable due to flushing or pruritus but is hemodynamically stable and not experiencing chest pain or muscle spasm), we suggest treating with an H1 antihistamine (Grade 2C). We administer diphenhydramine (50 mg orally or intravenously). We usually restart the infusion at one-half of the previous rate.
- For severe reactions (eg, muscle spasms, chest pain, or hypotension), in addition to stopping the infusion, we suggest treatment with both H1 and H2 antihistamines (Grade 2C). We administer diphenhydramine (50 mg intravenously) and famotidine (20 mg intravenously). Intravenous fluids may be needed for hypotension. We suggest infusing any subsequent doses of vancomycin over four hours with continuous hemodynamic monitoring during infusions.
- Prevention of repeat reactions – For patients with a previous history of VIR who require vancomycin again, we recommend antihistamine premedication with at least an H1 antihistamine (Grade 1B). We suggest a combination of H1 and H2 antihistamines (Grade 2C). We administer the combination of diphenhydramine (50
mg orally) and famotidine (20 mg orally) one hour before infusion and infuse each vancomycin dose over four hours.
- Indications for desensitization – For patients with recurrent VIR despite premedication and slow infusion rates who absolutely require vancomycin in the future, we suggest desensitization (Grade 2C). Desensitization involves gradually reintroducing the culprit drug in serially increasing doses to induce a state of temporary clinical tolerance. There are several published protocols. We prefer a rapid protocol that allows the patient to receive a full dose of vancomycin within several hours ( table 5). (See ‘Desensitization’ above.)
- Rare anaphylaxis – Anaphylaxis in response to vancomycin administration is rare. Symptoms of anaphylaxis overlap with those of severe VIR, although wheezing, significant dyspnea, and angioedema are more suggestive of anaphylaxis ( table 4). Multiple prior vancomycin courses should raise concern about the potential for immunoglobulin E (IgE) mediated anaphylaxis. (See ‘Clinical manifestations’ above.)
- Treatment – For patients with anaphylaxis of any severity:
- The infusion should be stopped immediately and not restarted.
- Epinephrine should be administered promptly, at the doses specified for adults ( table 2) or children ( table 3). (See ‘Acute management’ above and “Anaphylaxis: Emergency treatment”, section on ‘Immediate management’.)
- Future management options – For patients with past anaphylaxis to vancomycin, an alternative drug should be used whenever possible. For patients with serious infections that cannot be adequately treated with alternate antibiotics, we suggest vancomycin desensitization (Grade 2C). (See ‘Desensitization’ above.)
- Treatment – For patients with anaphylaxis of any severity:
- Other rare types of reactions – Other rare forms of vancomycin hypersensitivity include drug reaction with eosinophilia and systemic symptoms (DRESS)/drug- induced hypersensitivity syndrome, linear immunoglobulin A (IgA) bullous dermatosis (LABD) (
 picture 1), and immune-mediated hematologic and kidney disorders. The drug must be discontinued if these occur. Desensitization in patients with these reactions is not effective and may be dangerous. (See ‘Other forms of hypersensitivity’ above.)
picture 1), and immune-mediated hematologic and kidney disorders. The drug must be discontinued if these occur. Desensitization in patients with these reactions is not effective and may be dangerous. (See ‘Other forms of hypersensitivity’ above.)
REFERENCES
- Austin JP, Foster BA, Empey A. Replace Red Man Syndrome With Vancomycin Flushing Reaction. Hosp Pediatr 2020; 10:623.
- Alvarez-Arango S, Ogunwole SM, Sequist TD, et al. Vancomycin Infusion Reaction – Moving beyond “Red Man Syndrome”. N Engl J Med 2021; 384:1283.
- Nagahama Y, VanBeek MJ, Greenlee JDW. Red man syndrome caused by vancomycin powder. J Clin Neurosci 2018; 50:149.
- Rao S, Kupfer Y, Pagala M, et al. Systemic absorption of oral vancomycin in patients with Clostridium difficile infection. Scand J Infect Dis 2011; 43:386.
- Bergeron L, Boucher FD. Possible red-man syndrome associated with systemic absorption of oral vancomycin in a child with normal renal function. Ann Pharmacother 1994; 28:581.
- Symons NL, Hobbes AF, Leaver HK. Anaphylactoid reactions to vancomycin during anaesthesia: two clinical reports. Can Anaesth Soc J 1985; 32:178.
- Hepner DL, Castells MC. Anaphylaxis during the perioperative period. Anesth Analg 2003; 97:1381.
- Glicklich D, Figura I. Vancomycin and cardiac arrest. Ann Intern Med 1984; 101:880.
- Hicks RW, Hernandez J. Perioperative pharmacology: a focus on vancomycin. AORN J 2011; 93:593.
- Foer D, Wien M, Karlson EW, et al. Patient Characteristics Associated With Reactions to Mrgprx2-Activating Drugs in an Electronic Health Record-Linked Biobank. J Allergy Clin Immunol Pract 2023; 11:492.
- Mayhew JF, Deutsch S. Cardiac arrest following administration of vancomycin. Can Anaesth Soc J 1985; 32:65.
- Veien M, Szlam F, Holden JT, et al. Mechanisms of nonimmunological histamine and tryptase release from human cutaneous mast cells. Anesthesiology 2000; 92:1074.
- Horinouchi Y, Abe K, Kubo K, Oka M. Mechanisms of vancomycin-induced histamine release from rat peritoneal mast cells. Agents Actions 1993; 40:28.
- McNeil BD, Pundir P, Meeker S, et al. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 2015; 519:237.
- Navinés-Ferrer A, Serrano-Candelas E, Lafuente A, et al. MRGPRX2-mediated mast cell
response to drugs used in perioperative procedures and anaesthesia. Sci Rep 2018; 8:11628.
- Healy DP, Sahai JV, Fuller SH, Polk RE. Vancomycin-induced histamine release and “red man syndrome”: comparison of 1- and 2-hour infusions. Antimicrob Agents Chemother 1990; 34:550.
- Wallace MR, Mascola JR, Oldfield EC 3rd. Red man syndrome: incidence, etiology, and prophylaxis. J Infect Dis 1991; 164:1180.
- Adrouny A, Meguerditchian S, Koo CH, et al. Agranulocytosis related to vancomycin therapy. Am J Med 1986; 81:1059.
- O’Sullivan TL, Ruffing MJ, Lamp KC, et al. Prospective evaluation of red man syndrome in patients receiving vancomycin. J Infect Dis 1993; 168:773.
- Renz CL, Thurn JD, Finn HA, et al. Oral antihistamines reduce the side effects from rapid vancomycin infusion. Anesth Analg 1998; 87:681.
- Newfield P, Roizen MF. Hazards of rapid administration of vancomycin. Ann Intern Med 1979; 91:581.
- Polk RE, Healy DP, Schwartz LB, et al. Vancomycin and the red-man syndrome: pharmacodynamics of histamine release. J Infect Dis 1988; 157:502.
- Levy JH, Marty AT. Vancomycin and adverse drug reactions. Crit Care Med 1993; 21:1107.
- Wong JT, Ripple RE, MacLean JA, et al. Vancomycin hypersensitivity: synergism with narcotics and “desensitization” by a rapid continuous intravenous protocol. J Allergy Clin Immunol 1994; 94:189.
- Renz CL, Thurn JD, Finn HA, et al. Antihistamine prophylaxis permits rapid vancomycin infusion. Crit Care Med 1999; 27:1732.
- Hassaballa H, Mallick N, Orlowski J. Vancomycin anaphylaxis in a patient with vancomycin-induced red man syndrome. Am J Ther 2000; 7:319.
- Anne’ S, Middleton E Jr, Reisman RE. Vancomycin anaphylaxis and successful desensitization. Ann Allergy 1994; 73:402.
- Chopra N, Oppenheimer J, Derimanov GS, Fine PL. Vancomycin anaphylaxis and successful desensitization in a patient with end stage renal disease on hemodialysis by maintaining steady antibiotic levels. Ann Allergy Asthma Immunol 2000; 84:633.
- Fisher MM, Baldo BA. Mast cell tryptase in anaesthetic anaphylactoid reactions. Br J Anaesth 1998; 80:26.
- Renz CL, Laroche D, Thurn JD, et al. Tryptase levels are not increased during vancomycin-induced anaphylactoid reactions. Anesthesiology 1998; 89:620.
- Laroche D, Vergnaud MC, Sillard B, et al. Biochemical markers of anaphylactoid reactions to drugs. Comparison of plasma histamine and tryptase. Anesthesiology 1991; 75:945.
- Schwartz LB, Yunginger JW, Miller J, et al. Time course of appearance and disappearance of human mast cell tryptase in the circulation after anaphylaxis. J Clin Invest 1989; 83:1551.
- Matsson P, Enander I, Andersson AS, et al. Evaluation of mast cell activation (tryptase) in two patients suffering from drug-induced hypotensoid reactions. Agents Actions 1991; 33:218.
- Ordoqui E, Zubeldia JM, Aranzábal A, et al. Serum tryptase levels in adverse drug reactions. Allergy 1997; 52:1102.
- Schwartz LB, Bradford TR, Rouse C, et al. Development of a new, more sensitive immunoassay for human tryptase: use in systemic anaphylaxis. J Clin Immunol 1994; 14:190.
- Minhas JS, Wickner PG, Long AA, et al. Immune-mediated reactions to vancomycin: A systematic case review and analysis. Ann Allergy Asthma Immunol 2016; 116:544.
- Lin RY. Desensitization in the management of vancomycin hypersensitivity. Arch Intern Med 1990; 150:2197.
- Perencevich EN, Weller PF, Samore MH, Harris AD. Benefits of negative penicillin skin test results persist during subsequent hospital admissions. Clin Infect Dis 2001; 32:317.
- Frigas E, Park MA, Narr BJ, et al. Preoperative evaluation of patients with history of allergy to penicillin: comparison of 2 models of practice. Mayo Clin Proc 2008; 83:651.
- Park M, Markus P, Matesic D, Li JT. Safety and effectiveness of a preoperative allergy clinic in decreasing vancomycin use in patients with a history of penicillin allergy. Ann Allergy Asthma Immunol 2006; 97:681.
- Gilmore JF, Kim M, LaSalvia MT, Mahoney MV. Treatment of enterococcal peritonitis with intraperitoneal daptomycin in a vancomycin-allergic patient and a review of the
literature. Perit Dial Int 2013; 33:353.
- Blaskovich MAT, Hansford KA, Butler MS, et al. Developments in Glycopeptide Antibiotics. ACS Infect Dis 2018; 4:715.
- Villavicencio AT, Hey LA, Patel D, Bressler P. Acute cardiac and pulmonary arrest after infusion of vancomycin with subsequent desensitization. J Allergy Clin Immunol 1997; 100:853.
- Kitazawa T, Ota Y, Kada N, et al. Successful vancomycin desensitization with a combination of rapid and slow infusion methods. Intern Med 2006; 45:317.
- Kwon HS, Chang YS, Jeong YY, et al. A case of hypersensitivity syndrome to both vancomycin and teicoplanin. J Korean Med Sci 2006; 21:1108.
- Ben m’rad M, Leclerc-Mercier S, Blanche P, et al. Drug-induced hypersensitivity syndrome: clinical and biologic disease patterns in 24 patients. Medicine (Baltimore) 2009; 88:131.
- Wazny LD, Daghigh B. Desensitization protocols for vancomycin hypersensitivity. Ann Pharmacother 2001; 35:1458.
- Lerner A, Dwyer JM. Desensitization to vancomycin. Ann Intern Med 1984; 100:157.
- Castells M. Desensitization for drug allergy. Curr Opin Allergy Clin Immunol 2006; 6:476.
- Sorensen SJ, Wise SL, al-Tawfiq JA, et al. Successful vancomycin desensitization in a patient with end-stage renal disease and anaphylactic shock to vancomycin. Ann Pharmacother 1998; 32:1020.
- Clayman MD, Capaldo RA. Vancomycin allergy presenting as fever of unknown origin. Arch Intern Med 1989; 149:1425.
- Rocha JL, Kondo W, Baptista MI, et al. Uncommon vancomycin-induced side effects. Braz J Infect Dis 2002; 6:196.
- Tamagawa-Mineoka R, Katoh N, Nara T, et al. DRESS syndrome caused by teicoplanin and vancomycin, associated with reactivation of human herpesvirus-6. Int J Dermatol 2007; 46:654.
- Vauthey L, Uçkay I, Abrassart S, et al. Vancomycin-induced DRESS syndrome in a female patient. Pharmacology 2008; 82:138.
- Boet S, Noblet C, Haas-Hubscher C, et al. Severe vancomycin-induced drug rash with
eosinophilia and systemic symptoms syndrome imitating septic shock. Eur J Anaesthesiol 2009; 26:791.
- Wolfson AR, Zhou L, Li Y, et al. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Syndrome Identified in the Electronic Health Record Allergy Module. J Allergy Clin Immunol Pract 2019; 7:633.
- Konvinse KC, Trubiano JA, Pavlos R, et al. HLA-A*32:01 is strongly associated with vancomycin-induced drug reaction with eosinophilia and systemic symptoms. J Allergy Clin Immunol 2019; 144:183.
- Hung YP, Lee NY, Chang CM, et al. Tolerability of teicoplanin in 117 hospitalized adults with previous vancomycin-induced fever, rash, or neutropenia: a retrospective chart review. Clin Ther 2009; 31:1977.
- An SY, Hwang EK, Kim JH, et al. Vancomycin-associated spontaneous cutaneous adverse drug reactions. Allergy Asthma Immunol Res 2011; 3:194.
- Perrin-Lamarre A, Petitpain N, Trechot P, et al. [Glycopeptide-induced cutaneous adverse reaction: results of an immunoallergic investigation in eight patients]. Ann Dermatol Venereol 2010; 137:101.
- Prey S, Sparsa A, Boumediene A, et al. [Cutaneous drug reactions induced by glycopeptides]. Med Mal Infect 2007; 37:270.
- Cavalcanti AB, Goncalves AR, Almeida CS, et al. Teicoplanin versus vancomycin for proven or suspected infection. Cochrane Database Syst Rev 2010; :CD007022.
- Nousari HC, Costarangos C, Anhalt GJ. Vancomycin-associated linear IgA bullous dermatosis. Ann Intern Med 1998; 129:507.
- Bernstein EF, Schuster M. Linear IgA bullous dermatosis associated with vancomycin. Ann Intern Med 1998; 129:508.
- Bitman LM, Grossman ME, Ross H. Bullous drug eruption treated with amputation. A challenging case of vancomycin-induced linear IgA disease. Arch Dermatol 1996; 132:1289.
- Richards SS, Hall S, Yokel B, Whitmore SE. A bullous eruption in an elderly woman. Vancomycin-associated linear IgA dermatosis (LAD). Arch Dermatol 1995; 131:1447.
- Neughebauer BI, Negron G, Pelton S, et al. Bullous skin disease: an unusual allergic reaction to vancomycin. Am J Med Sci 2002; 323:273.
- Solky BA, Pincus L, Horan RF. Vancomycin-induced linear IgA bullous dermatosis:
morphology is a key to diagnosis. Cutis 2004; 73:65.
- Onodera H, Mihm MC Jr, Yoshida A, Akasaka T. Drug-induced linear IgA bullous dermatosis. J Dermatol 2005; 32:759.
- Mofid MZ, Costarangos C, Bernstein B, et al. Drug-induced linear immunoglobulin A bullous disease that clinically mimics toxic epidermal necrolysis. J Burn Care Rehabil 2000; 21:246.
- Coelho S, Tellechea O, Reis JP, et al. Vancomycin-associated linear IgA bullous dermatosis mimicking toxic epidermal necrolysis. Int J Dermatol 2006; 45:995.
- Park JS, Hamilton CD, Patel S, et al. Linear Immunoglobulin A (IgA) Bullous Dermatosis Mimicking Stevens-Johnson Syndrome. Cureus 2022; 14:e30309.
- Billet SE, Kortuem KR, Gibson LE, El-Azhary R. A morbilliform variant of vancomycin- induced linear IgA bullous dermatosis. Arch Dermatol 2008; 144:774.
- Laurencin CT, Horan RF, Senatus PB, et al. Stevens-Johnson-type reaction with vancomycin treatment. Ann Pharmacother 1992; 26:1520.
- Alexander II, Greenberger PA. Vancomycin-induced Stevens-Johnson syndrome. Allergy Asthma Proc 1996; 17:75.
- Neal D, Morton R, Bailie GR, Waldek S. Exfoliative reaction to vancomycin. Br Med J (Clin Res Ed) 1988; 296:137.
- Vidal C, González Quintela A, Fuente R. Toxic epidermal necrolysis due to vancomycin. Ann Allergy 1992; 68:345.
- Gilmore ES, Friedman JS, Morrell DS. Extensive fixed drug eruption secondary to vancomycin. Pediatr Dermatol 2004; 21:600.
- Felix-Getzik E, Sylvia LM. Vancomycin-induced leukocytoclastic vasculitis. Pharmacotherapy 2009; 29:846.
- Marik PE, Ferris N. Delayed hypersensitivity reaction to vancomycin. Pharmacotherapy 1997; 17:1341.
- Smith PF, Taylor CT. Vancomycin-induced neutropenia associated with fever: similarities between two immune-mediated drug reactions. Pharmacotherapy 1999; 19:240.
- Von Drygalski A, Curtis BR, Bougie DW, et al. Vancomycin-induced immune thrombocytopenia. N Engl J Med 2007; 356:904.
- Christie DJ, van Buren N, Lennon SS, Putnam JL. Vancomycin-dependent antibodies associated with thrombocytopenia and refractoriness to platelet transfusion in patients with leukemia. Blood 1990; 75:518.
- Marraffa J, Guharoy R, Duggan D, et al. Vancomycin-induced thrombocytopenia: a case proven with rechallenge. Pharmacotherapy 2003; 23:1195.
- Reis AG, Grisi SJ. Adverse effects of vancomycin in children: a review of 22 cases. Sao Paulo Med J 1997; 115:1452.
- Black E, Lau TT, Ensom MH. Vancomycin-induced neutropenia: is it dose- or duration- related? Ann Pharmacother 2011; 45:629.
- Labbus K, Junkmann JK, Perka C, et al. Antibiotic-induced fever in orthopaedic patients-a diagnostic challenge. Int Orthop 2018; 42:1775.
- Hazlewood KA, Brouse SD, Pitcher WD, Hall RG. Vancomycin-associated nephrotoxicity: grave concern or death by character assassination? Am J Med 2010; 123:182.e1.
- Vandecasteele SJ, De Vriese AS. Recent changes in vancomycin use in renal failure. Kidney Int 2010; 77:760.
- Welch HK, Kellum JA, Kane-Gill SL. Drug-Associated Acute Kidney Injury Identified in the United States Food and Drug Administration Adverse Event Reporting System Database. Pharmacotherapy 2018; 38:785.
- Wai AO, Lo AM, Abdo A, Marra F. Vancomycin-induced acute interstitial nephritis. Ann Pharmacother 1998; 32:1160.
- Salazar MN, Matthews M, Posadas A, et al. Biopsy proven interstitial nephritis following treatment with vancomycin: a case report. Conn Med 2010; 74:139.
- Codding CE, Ramseyer L, Allon M, et al. Tubulointerstitial nephritis due to vancomycin. Am J Kidney Dis 1989; 14:512.
- Wood MJ. Comparative safety of teicoplanin and vancomycin. J Chemother 2000; 12 Suppl 5:21.
- Svetitsky S, Leibovici L, Paul M. Comparative efficacy and safety of vancomycin versus teicoplanin: systematic review and meta-analysis. Antimicrob Agents Chemother 2009; 53:4069.
- Wilson AP. Comparative safety of teicoplanin and vancomycin. Int J Antimicrob Agents 1998; 10:143.
- Khurana C, de Belder MA. Red-man syndrome after vancomycin: potential cross- reactivity with teicoplanin. Postgrad Med J 1999; 75:41.
- Sahai J, Healy DP, Shelton MJ, et al. Comparison of vancomycin- and teicoplanin- induced histamine release and “red man syndrome”. Antimicrob Agents Chemother 1990; 34:765.
- Sice PJA, Ford S, Whyte AF, Greig JR. Teicoplanin hypersensitivity during anaesthesia and surgery. Br J Clin Pharmacol 2019; 85:854.
- Azamgarhi T, Shah A, Warren S. Teicoplanin anaphylaxis associated with surgical prophylaxis. Br J Clin Pharmacol 2018; 84:1038.
- Savic LC, Garcez T, Hopkins PM, et al. Teicoplanin allergy – an emerging problem in the anaesthetic allergy clinic. Br J Anaesth 2015; 115:595.
- Marshall C, Street A, Galbraith K. Glycopeptide-induced vasculitis–cross-reactivity between vancomycin and teicoplanin. J Infect 1998; 37:82.
- Henson KE, Levine MT, Wong EA, Levine DP. Glycopeptide antibiotics: evolving resistance, pharmacology and adverse event profile. Expert Rev Anti Infect Ther 2015; 13:1265.
- Hahn AW, Jain R, Spach DH. New Approaches to Antibiotic Use and Review of Recently Approved Antimicrobial Agents. Med Clin North Am 2016; 100:911.
- Ishizuka KT, Tran TK, Ayars AG, et al. Graded Dalbavancin Challenge in a Patient With Severe Vancomycin Hypersensitivity Reaction. Clin Infect Dis 2020; 70:1230.
Topic 2083 Version 24.0
© 2023 UpToDate, Inc. All rights reserved.
-
Therapeutic Monitoring of Vancomycin for Serious Methicillin-resistant Staphylococcus aureus Infections: A Revised Consensus Guideline and Review by the American Society of Health-system Pharmacists, the Infectious Diseases Society of America, the Pediatric

 Clinical Infectious Diseases
Clinical Infectious DiseasesIDS A FEA TURE S
Therapeutic Monitoring of Vancomycin for Serious Methicillin-resistant Staphylococcus aureus Infections: A Revised Consensus Guideline and Review by the American Society of Health-system Pharmacists, the Infectious Diseases Society of America, the Pediatric
Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists
Michael J. Rybak,1,2,3 Jennifer Le,4 Thomas P. Lodise,5 Donald P. Levine,2,3 John S. Bradley,6,7 Catherine Liu,8,9 Bruce A. Mueller,10 Manjunath P. Pai,10 Annie Wong-Beringer,11 John C. Rotschafer,12 Keith A. Rodvold,13 Holly D. Maples,14 and Benjamin Lomaestro15
1Anti-Infective Research Laboratory, Department of Pharmacy Practice, Eugene Applebaum College of Pharmacy and Health Sciences, Wayne State University, Detroit, Michigan, USA, 2School of Medicine, Wayne State University, Detroit, Michigan, USA, 3Detroit Receiving Hospital, Detroit, Michigan, USA, 4Skaggs School of Pharmacy and Pharmaceutical Sciences, University of California, San Diego, La Jolla, California, USA, 5Albany College of Pharmacy and Health Sciences, Albany, New York, USA, 6Department of Pediatrics, Division of Infectious Diseases, University of California, San Diego, La Jolla, California, USA, 7Rady Children’s Hospital San Diego, San Diego, California, USA, 8Division of Allergy and Infectious Diseases, University of Washington, Seattle, Washington, USA, 9Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Research Center, Seattle, Washington, USA, 10University of Michigan College of Pharmacy, Ann Arbor, Michigan, USA, 11University of Southern California School of Pharmacy, Los Angeles, California, USA, 12University of Minnesota College of Pharmacy, Minneapolis, Minnesota, USA, 13University of Illinois College of Pharmacy, Chicago, Illinois, USA, 14University of Arkansas for Medical Sciences College of Pharmacy and Arkansas Children’s Hospital, Little Rock, Arkansas, USA, and 15Albany Medical Center Hospital, Albany, New York, USA
Recent clinical data on vancomycin pharmacokinetics and pharmacodynamics suggest a reevaluation of current dosing and moni- toring recommendations. The previous 2009 vancomycin consensus guidelines recommend trough monitoring as a surrogate marker for the target area under the curve over 24 hours to minimum inhibitory concentration (AUC/MIC). However, recent data suggest that trough monitoring is associated with higher nephrotoxicity. This document is an executive summary of the new van- comycin consensus guidelines for vancomycin dosing and monitoring. It was developed by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists vancomycin consensus guidelines committee. These consensus guidelines recommend an AUC/MIC ratio of 400–600 mg*hour/L (assuming a broth microdilution MIC of 1 mg/L) to achieve clinical efficacy and ensure safety for patients being treated for serious methicillin-resistant Staphylococcus aureus infections.
Keywords. vancomycin consensus guidelines; vancomycin; pharmacokinetics and pharmacodynamics; target attainment; nephrotoxicity.
EXECUTIVE SUMMARY
The revised vancomycin consensus guidelines for dosing and monitoring vancomycin is an updated version of the 2009 guidelines developed by the American Society of Health- Systems Pharmacists, the Infectious Diseases Society of
America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists vancomycin guidelines committee. The following is an executive sum- mary of key recommendations and grading system used for this document (Tables 1, 2) [1, 2].
Despite more than 61 years of clinical use of vancomycin, knowledge gaps regarding the most appropriate approach for
optimizing therapy and minimizing toxicity still exist. The area
Received 12 March 2020; editorial decision 12 March 2020; published online July 13, 2020. Correspondence: M. J. Rybak, Anti-Infective Research Laboratory, Department of Pharmacy
Downloaded from https://academic.oup.com/cid/article/71/6/1361/5870833 by guest on 04 December 2023
Practice, Eugene Applebaum College of Pharmacy, Wayne State University, 259 Mack Ave, Detroit, MI 48201 ([email protected]).
Clinical Infectious Diseases® 2020;71(6):1361–4
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: [email protected].
DOI: 10.1093/cid/ciaa303
under the curve over 24 hours to minimum inhibitory con- centration ratio (AUC/MIC) has been documented as the pri- mary pharmacokinetic/pharmacodynamic (PK/PD) target for glycopeptides, including vancomycin. The previous consensus guidelines in 2009 recommended the use of trough moni- toring (target 15–20 mg/L) as a surrogate marker of the AUC/
Table 1. Grading System for Recommendations Based on Quality of Evidence
Downloaded from https://academic.oup.com/cid/article/71/6/1361/5870833 by guest on 04 December 2023
Category and Grade Definition
Strength of recommendation
A Good evidence to support a recommendation for or against use
B Moderate evidence to support a recommendation for or against use
C Poor evidence to support a recommendation
Quality of evidence
I Evidence from 1 or more properly randomized controlled trials
II
Evidence from 1 or more well-designed clinical trials, without randomization; from cohort or case-controlled analytic studies (preferably from > 1 center); from multiple time-series; or from dramatic results from uncontrolled experiments
III Evidence from opinions of respected authorities, based on clinical experience, descriptive studies, or reports of expert committees
Adapted from the Canadian Task Force on the Periodic Health Examination [2].
Table 2. Primary Recommendations for Vancomycin Dosing and Therapeutic Drug Monitoring
- ADULTS AND PEDIATRICS
1. In patients with suspected or definitive serious MRSA infections, an individualized target of the AUC/MICBMD ratio of 400 to 600 (assuming a vancomycin MICBMD of 1 mg/L) should be advocated to achieve clinical efficacy while improving patient safety (A-II).
2. When transitioning to AUC/MIC monitoring, clinicians should conservatively target AUCs for patients with suspected or documented serious infections due to MRSA assuming a vancomycin MICBMD of 1 mg/L or less at most institutions. Given the importance of early, appropriate therapy, vancomycin targeted exposure should be achieved early during the course of therapy, preferably within the first 24 to 48 hours (A-II). As such, the use of Bayesian-derived AUC
monitoring may be prudent in these cases since it doesn’t require steady-state serum vancomycin concentrations to allow for early assessment of AUC target attainment.
3. Trough-only monitoring, with target between 15 and 20 mg/L, is no longer recommended based on efficacy and nephrotoxicity data in patients with serious infections due to MRSA (A-II). There is insufficient evidence to provide recommendations on whether trough-only or AUC-guided vancomycin monitoring should be used among patients with noninvasive MRSA or other infections.
4. Vancomycin monitoring is recommended for patients receiving vancomycin for serious MRSA infections to achieve sustained targeted AUC (assuming a MICBMD of 1 mg/L, unless it is known to be greater or less than 1 mg/L by BMD). Independent of MRSA infection, vancomycin monitoring is also recom- mended for all patients at high risk of nephrotoxicity (eg, critically ill patients receiving concurrent nephrotoxins), patients with unstable (ie, deteriorating or significantly improving) renal function, and those receiving prolonged courses of therapy (more than 3–5 days). We suggest the frequency of monitoring be based on clinical judgement; frequent or daily monitoring may be prudent for hemodynamically unstable patients (eg, end-stage renal disease) and once- weekly monitoring for hemodynamically stable patients (B-II).
5. Based on current national vancomycin susceptibility surveillance data, under most circumstances for empiric dosing, the vancomycin MIC should be as- sumed to be 1 mg/L. When the MICBMD is > 1 mg/L, the probability of achieving an AUC/MIC ≥ 400 target is unlikely with conventional dosing; higher doses may risk unnecessary toxicity and the decision to change therapy should be based on clinical judgment. In addition, when MICBMD < 1 mg/L, we do not rec- ommend decreasing the dose to achieve the AUC/MIC target. It is important to note the limitations in automated susceptibility testing methods, including the lack of precision and variability in MIC results depending on method used (B-II).
6. The PK of continuous infusion suggest that such regimens may be a reasonable alternative to conventional intermittent infusion dosing when the AUC target cannot be achieved (B-II).
7. Incompatibility with vancomycin and other drugs commonly coadministered in the ICU requires the use of independent lines or multiple catheters when vancomycin is being considered for continuous infusion (A-III).
- ADULTS
- Given the narrow vancomycin AUC range for therapeutic effect and minimal AKI, the most accurate and optimal way to manage vancomycin dosing should be through AUC-guided dosing and monitoring (A-II). We recommend to accomplish this in 1 of 2 ways.
- One approach relies on the collection of 2 concentrations (obtained near steady-state, postdistributional peak concentration at 1–2 hours after infusion and trough at end of dosing interval) preferably but not required during the same dosing interval (if possible) and utilizing first-order PK equations to esti- mate the AUC (A-II).
- The preferred approach to monitor AUC involves the use of Bayesian software programs, embedded with a PK model based on richly sampled vanco- mycin data as the Bayesian prior, to optimize the delivery of vancomycin based on the collection of 1 or 2 vancomycin concentrations, with at least 1 trough. It is preferred to obtain 2 PK samples (ie, 1–2 hours postinfusion and at end of dosing interval) to estimate the AUC with the Bayesian approach (A-II). A trough concentration alone may be sufficient to estimate the AUC with the Bayesian approach in some patients, but more data are needed across different patient populations to confirm viability of using trough only data (B-II).
9. Doses of 15 to 20 mg/kg (based on actual body weight) administered every 8–12 hours as an intermittent infusion are recommended for most patients with
normal renal function when assuming MICBMD of 1 mg/L (A-II). In patients with normal renal function, these doses may not achieve therapeutic AUC/MIC target when the MIC is 2 mg/L.
10. Continuous infusion: Based on current available data, a loading dose of 15-20 mg/kg, followed by daily maintenance CI of 30–40 mg/kg up to 60 mg/kg, to achieve target steady-state concentration of 20–25 mg/L may be considered for critically ill patients (B-II). AUC24 can be simply calculated when multiplying steady-state concentration (ie, desired therapeutic range of 20–25 mg/L throughout entire dosing interval) by a factor of 24 (B-II). Attaining the desired drug exposure may be more readily accomplished given the ease of sampling time and dosage adjustment by changing the rate of infusion which is a highly de- sirable feature in critically ill patients (B-II).
11. The risk of developing nephrotoxicity with continuous infusion appears to be similar or lower compared to intermittent dosing when targeting steady-state concentration 15–25 mg/L and trough 10–20 mg/L, respectively (B-II). Definitive studies are needed to compare drug exposure based on measured AUC24 and factors that predispose to development of nephrotoxicity such as receipt of concomitant nephrotoxins, diuretics, and/or vasopressor therapy in patients receiving continuous infusion vs intermittent infusion of vancomycin.
Table 2. Continued
12. In order to achieve rapid attainment of targeted concentrations in critically ill patients with suspected or documented serious MRSA infections, a loading dose of 20–35 mg/kg can be considered for intermittent administration of vancomycin (B-II). Loading doses should be based on actual body weight and not exceed 3000 mg. More intensive and early therapeutic monitoring should also be performed in obese patients (B-II).
13. Adult obesity: A vancomycin loading dose of 20–25 mg/kg using actual body weight with a maximum of 3000 mg may be considered in obese adult pa- tients with serious infections (B-II). Empiric maintenance doses for most obese patients usually do not exceed 4500 mg/day, depending on their renal function (B-II). Early and frequent monitoring of AUC exposure is recommended for dose adjustment, especially when empiric doses exceed 4000 mg/day (A-II).
14. Intermittent hemodialysis: Since efficacy data are unavailable for AUC < 400 mg*h/L, monitoring based on predialysis serum concentrations and extrapo- lating these values to estimate AUC is most practical. Maintaining predialysis concentrations between 15 and 20 mg/L are likely to achieve the AUC of 400-600 mg*h/L in the previous 24 hours (C-III). Predialysis serum concentration monitoring should be performed not less than weekly and should drive
subsequent dosing rather than a strict weight-based recommendation, although these recommended doses provide a useful starting point until serum con- centrations have been determined (B-II).
15. Hybrid dialysis therapies (eg slow-low efficiency dialysis): Loading doses of 20–25 mg/kg actual body weight should be used, recognizing that these hybrid dialysis therapies efficiently remove vancomycin (B-III). Initial doses should not be delayed to wait for a dialysis treatment to end. Maintenance doses of 15 mg/kg should be given after hybrid hemodialysis ends or during the final 60–90 minutes of dialysis, as is done with standard hemodialysis (B-III). Concentra- tion monitoring should guide further maintenance doses.
16. Continuous renal replacement therapies: Loading doses of 20–25 mg/kg by actual body weight should be used in patients receiving CRRT at conventional, KDIGO-recommended effluent rates of 20-25 mL/kg/h (B-II). Initial maintenance dosing for CRRT with effluent rates of 20–25 mL/kg/h should be 7.5–10 mg/ kg every 12 hours (B-II). Maintenance dose and dosing interval should be based on serum concentration monitoring, which should be conducted within
the first 24 hours to ensure AUC/MIC targets are met. In fluid-overloaded patients, doses may be reduced as patients become euvolemic and drug Vd decreases. The use of continuous infusion vancomycin in patients receiving CRRT appears to be growing, and could be used in place of intermittent vanco- mycin dosing, especially when high CRRT ultrafiltrate/dialysate flow rates are employed (B-II).
- PEDIATRICS
17. Based on an AUC target of 400 mg*h/L (but potentially up to 600 mg*h/L assuming MIC of ≤ 1 mg/L) from adult data, the initial recommended vancomycin dosage for children with normal renal function and suspected serious MRSA infections is 60–80 mg/kg/day, divided every 6 to 8 hours, for children ages 3 months and older (A-II).
18. The maximum empiric daily dose is usually 3600 mg/day in children with adequate renal function (C-III). Most children generally should not require more than 3000 mg/day and doses should be adjusted based on observed concentrations to achieve the AUC/MIC target. Early monitoring of observed concen- trations is recommended when doses exceed 2000–3000 mg/day (A-III). Furthermore, close monitoring of observed concentrations and renal function is prudent in patients with poor or augmented renal clearance as resolution of their renal function may occur within the first 5 days of therapy.
19. AUC-guided therapeutic monitoring for vancomycin, preferably with Bayesian estimation, is suggested for all pediatric age groups, based on developmental changes of vancomycin CL documented from the newborn to the adolescent. Based on current available data, the suggestion for AUC-guided monitoring in pediatrics aligns with the approach for adults, including the application of Bayesian estimation for 1 trough concentration, or first-order PK equations with 2 concentrations (B-II). The Bayesian AUC-guided dosing strategy may be an optimal approach to individualize vancomycin therapy in pediatrics since it can incorporate varying ages, weights, and renal function. Both serum concentrations of vancomycin and renal function should be monitored since vancomycin CL and creatinine CL are not always well correlated in pediatrics. Furthermore, aggressive dosing to maintain target AUC exposure and decrease the risk of potential AKI in treatment of MRSA infection necessitates drug monitoring.
20. Therapeutic monitoring may begin within 24 to 48 hours of vancomycin therapy for serious MRSA infections in children, as in adults (B-III). Any delay in therapeutic monitoring should be based on severity of infection and clinical judgment. Dosing adjustment should be made for those with renal insufficiency, obesity, or for those receiving concurrent nephrotoxic drug therapy. Following the initial dose, dosing adjustment is important for those with acute renal insufficiency, but subsequent adjustment (particularly within the first 5 days of therapy) may be necessary for those experiencing recovery of renal function. Sustained or subsequent decreases in dosage may be needed, particularly for those with chronic renal insufficiency and those receiving concurrent nephro- toxic drug therapy (B-III).
21. Vancomycin exposure may be optimally maintained below the thresholds for AUC of 800 mg*h/L and trough concentrations of 15 mg/L to minimize AKI
(B-II). The safety of vancomycin above 80 mg/kg/day has not been prospectively evaluated. Avoiding vancomycin doses ≥ 100 mg/kg/day is suggested since they are likely to surpass these thresholds (B-III).
22. Insufficient data exist on which to base a recommendation for a loading dose among the nonobese pediatric population. Loading doses from adult studies may be considered, but further studies are needed to elucidate the appropriate dose for the various pediatric populations from the neonate to adolescent (C-III).
23. Pediatric obesity: Data suggest that obese children are likely to have vancomycin exposures that may be statistically greater than normal-weight children when doses are calculated on a mg/kg basis, but these differences are not known to be of sufficient clinical importance to suggest different mg/kg empiric vancomycin dosages in obese children at this time. Similar to nonobese children, obese children < 12 years old, compared with those ≥ 12 years, may re- quire higher mg/kg dose (B-II).
24. Pediatric obesity: Therapeutic monitoring is likely to be of particular value in obese children, both for therapeutic response and the risk of AKI. The specific recommendations for therapeutic monitoring in nonobese children may also apply for obese children (B-II). A loading dose of 20 mg/kg by total body weight is recommended in obese children (A-III).
25. Neonates: Doses recommended to achieve an AUC of 400 mg*h/L (assuming an MIC of 1 mg/L) in neonates and infants up to 3 months old range from 10 to 20 mg/kg every 8 to 48 hours, depending on postmenstrual age, weight, and SCr (A-II).
Abbreviations: AKI, acute kidney injury; AUC, area under the curve; AUC24, area under the curve over 24 hours; CL, clearance; CRRT, continuous renal replacement therapy; ICU, intensive care unit; KDIGO, Kidney Diseases Improving Global Outcomes; MICBMD, minimum inhibitory concentration, broth microdilution; MRSA, methicillin-resistant Staphylococcus aureus; PK, pharmacokinetics; SCr, serum creatinine; Vd, volume of distribution.
MIC (target 400 mg*hour/L) for ease of managing therapy and simplifying dose adjustments and monitoring. At that time, the primary reason for increasing the exposure of vancomycin via specific trough monitoring targets was to improve the
Downloaded from https://academic.oup.com/cid/article/71/6/1361/5870833 by guest on 04 December 2023
likelihood of achieving the AUC/MIC target of 400 mg*hour/L and thereby increasing efficacy. However, since the implemen- tation of these recommendations, there have been numerous reports of increased nephrotoxicity in adults and pediatrics
when trough level monitoring using these targets has been ap- plied. Recent PK/PD and toxicodynamic studies have demon- strated a significantly reduction in vancomycin exposure and nephrotoxicity rates without compromising outcomes when AUC/MIC monitoring has been employed vs traditional trough monitoring approaches.
Downloaded from https://academic.oup.com/cid/article/71/6/1361/5870833 by guest on 04 December 2023
When using AUC/MIC-guided empiric dosing, the MIC should be assumed to be 1 mg/L based on broth microdilution methods, extensive antibiotic susceptibility data, and the in- accuracies or variability of automated susceptibility testing (±1 log2 dilutions). Specific information regarding MIC eval- uation and automated susceptibility testing can be found under the MIC susceptibility section of the full guideline [1]. A target AUC between 400 and 600 mg*hour/L is suggested for methicillin-resistant Staphylococcus aureus (MRSA) invasive infections in adults and pediatrics based on clinical efficacy and safety data. These AUC targets should be achieved early in the course of therapy (24–48 hours) given the importance of early and appropriate therapy. Loading doses based on actual body weight are suggested for patients who are critically ill, requiring renal replacement therapy, or receiving continuous infusion therapy. Specific recommendations for patients with obesity on renal replacement therapy and, for the first time, pediatric pa- tients are now included in the revised guidelines [1].
It should be noted that almost all data available on vanco- mycin PK/PD and toxicodynamics have been derived from pa- tients who have been treated for serious infections of MRSA. Furthermore, the majority of the data have been derived from patients with complicated bloodstream infections. Therefore, caution should be applied when extrapolating this informa- tion to mild noninvasive infections or other bacterial species susceptible to vancomycin. These guidelines conclude that
AUC-guided dosing and monitoring is the most accurate and safest way to dose vancomycin. The recommendations in this document should not circumvent sound clinical judgment in managing patients who require vancomycin therapy. Specific details for each section of the document, including references, can be found in the primary publication [1].
Note
Potential conflicts of interest. T. P. L. is a board member of Motif; is a consultant for Paratek, Melinta, Merck, and Motif; has received grants from Merck and Motif; and is on the speaker’s bureaus of Melinta and Sunovion. B. A. M. has received grants from NxStage and Merck, and personal fees from Wolters-Kluwer. M. P. P. reports personal fees from Paratek and grants from Merck. K. A. R. is a consultant, on the speaker’s bureau, and/or served on advisory boards for the following companies: Achaogen, Allergan, Bayer, BLC USA, Entasis Therapeutics, GSK, Janssen Pharmaceuticals, Meiji, Melinta Therapeutics, Medicine Company, Merck, Motif Bio PLC, Nabriva Therapeutics, Qpex Biopharma, Rempex, Shionogi, Spero Therapeutics, Theravance Biopharma, Tetraphase, Wockhardt, and Zavante Therapeutics; and has received research grants and contracts (paid to the University of Illinois at Chicago) from Theravance Biopharma and Allergan. M. J. R. has received grants and personal fees from Allergan, Melinta, Merck, Motif, Paratek, Shionogi, and Tetraphase; personal fees from InsightRx; and grants from Contrafect. A. W.-B. has received grants from Merck and Allergan, and from Nabriva Therapeutics and Paratek Pharmaceuticals. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the con- tent of the manuscript have been disclosed.
References
- Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review of the American Society of Health-System Pharmacists, the Infectious Diseases Society by America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health-Syst Pharm 2020; 77:835–64. doi:10.1093/ajhp/zxaa036
- The periodic health examination. Canadian Task Force on the Periodic Health Examination. Can Med Assoc J 1979; 121:1193–254.
-
2023 American Heart Association and American Academy of Pediatrics Focused Update on Neonatal Resuscitation: An Update to the American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care

2023 American Heart Association and American Academy of Pediatrics Focused Update on Neonatal Resuscitation: An Update to the American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care
Nicole K. Yamada, MD, MS; Edgardo Szyld, MD, MSc, Co-Chair; Marya L. Strand, MD, MS; Emer Finan, MB, MEd; Jessica L. Illuzzi, MD, MS; Beena D. Kamath-Rayne, MD, MPH; Vishal S. Kapadia, MD, MSCS; Susan Niermeyer, MD, MPH; Georg M. Schmölzer, MD, PhD; Amanda Williams, RN, CNS, MSN; Gary M. Weiner, MD; Myra H. Wyckoff, MD; Henry C. Lee, MD, Co-Chair; on behalf of the American Heart Association and American Academy of Pediatrics
DOI: 10.1542/peds.2023-065030
Journal: Pediatrics
Article Type: Special Article
Citation: Yamada NK, Szyld E, Strand ML, et al. 2023 American Heart Association and American Academy of Pediatrics Focused Update on Neonatal Resuscitation: An Update to the American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Pediatrics. 2023; doi: 10.1542/peds.2023-065030
This is a prepublication version of an article that has undergone peer review and been accepted for publication but is not the final version of record. The journal is providing an early version of this article to expedite access to this information. The American Academy of Pediatrics, the editors, and authors are not responsible for inac- curate information and data described in this version.
©2023 American Academy of Pediatrics and American Heart Association Inc
Prepublication Release
AHA/AAP FOCUSED UPDATE
2023 American Heart Association and American Academy of Pediatrics Focused Update on Neonatal Resuscitation: An Update to the American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care
Nicole K. Yamada, MD, MS; Edgardo Szyld, MD, MSc, Co-Chair; Marya L. Strand, MD, MS; Emer Finan, MB, MEd; Jessica L. Illuzzi, MD, MS; Beena D. Kamath-Rayne, MD, MPH; Vishal S. Kapadia, MD, MSCS; Susan Niermeyer, MD, MPH; Georg M. Schmölzer, MD, PhD; Amanda Williams, RN, CNS, MSN; Gary M. Weiner, MD; Myra H. Wyckoff, MD; Henry C. Lee, MD, Co-Chair; on behalf of the American Heart Association and American Academy of Pediatrics
ABSTRACT: This 2023 focused update to the neonatal resuscitation guidelines is based on 4 systematic reviews recently completed under the direction of the International Liaison Committee on Resuscitation Neonatal Life Support Task Force. Systematic reviewers and content experts from this task force performed comprehensive reviews of the scientific literature on umbilical cord management in preterm, late preterm, and term newborn infants, and the optimal devices and interfaces used for administering positive-pressure ventilation during resuscitation of newborn infants. These recommendations provide new guidance on the use of intact umbilical cord milking, device selection for administering positive-pressure ventilation, and an additional primary interface for administering positive-pressure ventilation.
Key Words: AHA Scientific Statements ■ infant ■ infant, newborn ■ intermittent positive-pressure ventilation ■ laryngeal masks
- respiration, artificial ■ umbilical cord clamping ■ umbilical cord milking
TOP 10 TAKE-HOME MESSAGES FOR NEONATAL RESUSCITATION
- For term and late preterm newborn infants ≥34 weeks’ gestation who do not require resuscitation, delayed cord clamping (≥30 seconds) can be beneficial compared with early cord clamping (<30 seconds).
- For term and late preterm newborn infants ≥34 weeks’ gestation who do not require resuscitation, intact cord milking is not known to be beneficial compared with delayed cord clamping (≥30 seconds).
- For nonvigorous term and late preterm newborn infants (35–42 weeks’ gestation), intact cord milking may be reasonable compared with early cord clamping (<30 seconds).
- For preterm newborn infants <34 weeks’ gestation who do not require resuscitation, delaying cord clamping (≥30 seconds) can be beneficial compared with early cord clamping (<30 seconds).
- For preterm newborn infants between 28 and 34 weeks’ gestation who do not require resuscitation and in whom
delayed cord clamping cannot be performed, intact cord milking may be reasonable.
- For preterm newborn infants <28 weeks’ gestation, intact cord milking is not recommended.
- Effective positive-pressure ventilation is the priority in newborn infants who need support after birth.
- Using a T-piece resuscitator to deliver positive-pressure ventilation is preferred to the use of a self-inflating bag.
- Because both T-piece resuscitators and flow-inflating bags require a compressed gas source to function, a
self-inflating bag should be available as a backup in the event of compressed gas failure when using either of these
©2023 American Academy of Pediatrics and American Heart Association Inc
devices.
- Use of a supraglottic airway may be considered as the primary interface to administer positive-
pressure ventilation instead of a face mask for newborn infants delivered at ≥34 0/7 weeks’ gestation.
Abbreviations
AAP American Academy of Pediatrics AHA American Heart Association COR class of recommendation DCC delayed cord clamping ILCOR International Liaison Committee on Resuscitation LOE level of evidence PPV positive-pressure ventilation RCT randomized controlled trial - INTRODUCTION
Scope of the Guidelines
These guidelines are designed for North American health care practitioners caring for newborn infants who are looking for an up-to-date summary for clinical care, and for those who are seeking more in-depth information on these topics in resuscitation science and the gaps in current knowledge. This focused update is based on the systematic reviews of umbilical cord management in term and late preterm infants1 and preterm infants,2 and the devices and interfaces for administering positive-pressure ventilation (PPV).3,4 The findings of those systematic reviews are also reported in the “2021 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations”5 and the “2022 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations”6 from the International Liaison Committee on Resuscitation (ILCOR). The guidelines contained in this document serve as an update on these topics from the “2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care”7 and “Neonatal Life Support: 2020 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations” from the ILCOR Neonatal Life Support Task Force.8
Organization of the Writing Group
The Neonatal Life Support writing group includes neonatal physicians and nurses with backgrounds in clinical medicine, education, research, and public health. Volunteers with recognized expertise in neonatal resuscitation are nominated by the writing group co-chairs. Writing group members were selected by the American Heart Association (AHA) Emergency
©2023 American Academy of Pediatrics and American Heart Association Inc
Cardiovascular Care Science Subcommittee and the American Academy of Pediatrics (AAP) Executive Board and then
approved by the AHA Manuscript Oversight Committee.
The AHA and the AAP have rigorous conflict of interest policies and procedures to minimize the risk of bias or improper influence during the development of guidelines. Before their appointment, writing group members disclosed all relevant commercial relationships and other potential (including intellectual) conflicts. Writing group members whose research led to changes in guidelines were required to declare those conflicts during discussions and abstain from voting on those specific recommendations. These procedures are described more fully in “Part 2: Evidence Evaluation and Guidelines Development” of the 2020 guidelines.9
Appendix 1 of this document lists disclosure information and the writing group members’ relevant relationships with
industry.
Methodology and Evidence Review
Updated AHA/AAP guidelines for cardiopulmonary resuscitation and emergency cardiovascular care are developed in concert with ILCOR’s continuous evaluation of new resuscitation science.9 This 2023 focused update is based on 4 systematic reviews completed by the ILCOR Neonatal Life Support Task Force, which reviewed the science on umbilical cord management in preterm, late preterm, and term newborn infants1,2 and on devices and interfaces for administering PPV for newborn infants.3,4 The ILCOR Neonatal Life Support Task Force used the findings of these systematic reviews to draft treatment recommendations, which were posted online for public comment. The final wording has been published in the Consensus on Science With Treatment Recommendations summary documents from 2021 and 2022.5,6 Full details on the ILCOR systematic review process can be found in the 2022 publication.6 For this 2023 focused update, the Neonatal Life Support writing group analyzed and discussed the systematic reviews, carefully considered the treatment recommendations drafted by the ILCOR Neonatal Life Support Task Force, and incorporated new data published since the systematic reviews were completed. Guideline recommendations were drafted by designated writing group members and then reviewed and refined by all writing group members during regular meetings. The final recommendation wording was reviewed and approved by all writing group members.
Class of Recommendation and Level of Evidence
As with all AHA guidelines, each recommendation in this focused update is assigned a Class of Recommendation (COR) on the basis of the strength of the evidence, alternative treatment options, and the effect on patients and society (Table). The Level of Evidence (LOE) is based on the quality, quantity, relevance, and consistency of the available evidence. For each recommendation, the writing group discussed and approved specific wording of recommendations and the COR and LOE assignments. In determining the COR, the writing group considered the LOE and other factors, including systems issues, economic factors, and ethical factors such as equity, acceptability, and feasibility. These evidence review methods, including specific criteria used to determine COR and LOE, are described more fully in “Part 2: Evidence Evaluation and Guidelines Development” of the 2020 guidelines.9 The writing group members had final authority over and formally approved these recommendations.
The overall certainty of the evidence base for neonatal resuscitation science is low. Funding and support for high-certainty clinical trials is a significant need in neonatal resuscitation. Of the 8 recommendations in this focused update, no recommendations are supported by Level A evidence (high-quality evidence from >1 randomized controlled trial [RCT], or ≥1 RCTs corroborated by high-quality registry studies). Five recommendations are supported by Level B-R (randomized) evidence (moderate evidence from ≥1 RCTs) and 1 by Level B-N (nonrandomized) evidence. Two recommendations are based on Level C evidence supported by limited data; no recommendations are based on Level C evidence derived from expert opinion. Likewise, the strength of recommendations is weaker than optimal: no Class 1 (strong) recommendations, 3 Class 2a (moderate) recommendations, 3 Class 2b (weak) recommendations, and 2 Class 3: No Benefit recommendations are included in these guidelines. There are no recommendations designated Class 3: Harm.
©2023 American Academy of Pediatrics and American Heart Association Inc
Table. Applying the American College of Cardiology/American Heart AssociationClass of Recommendation and Level of Evidence to Clinical Strategies, Interventions, Treatments, or Diagnostic Testing in Patient Care* (Up- dated May 2019)
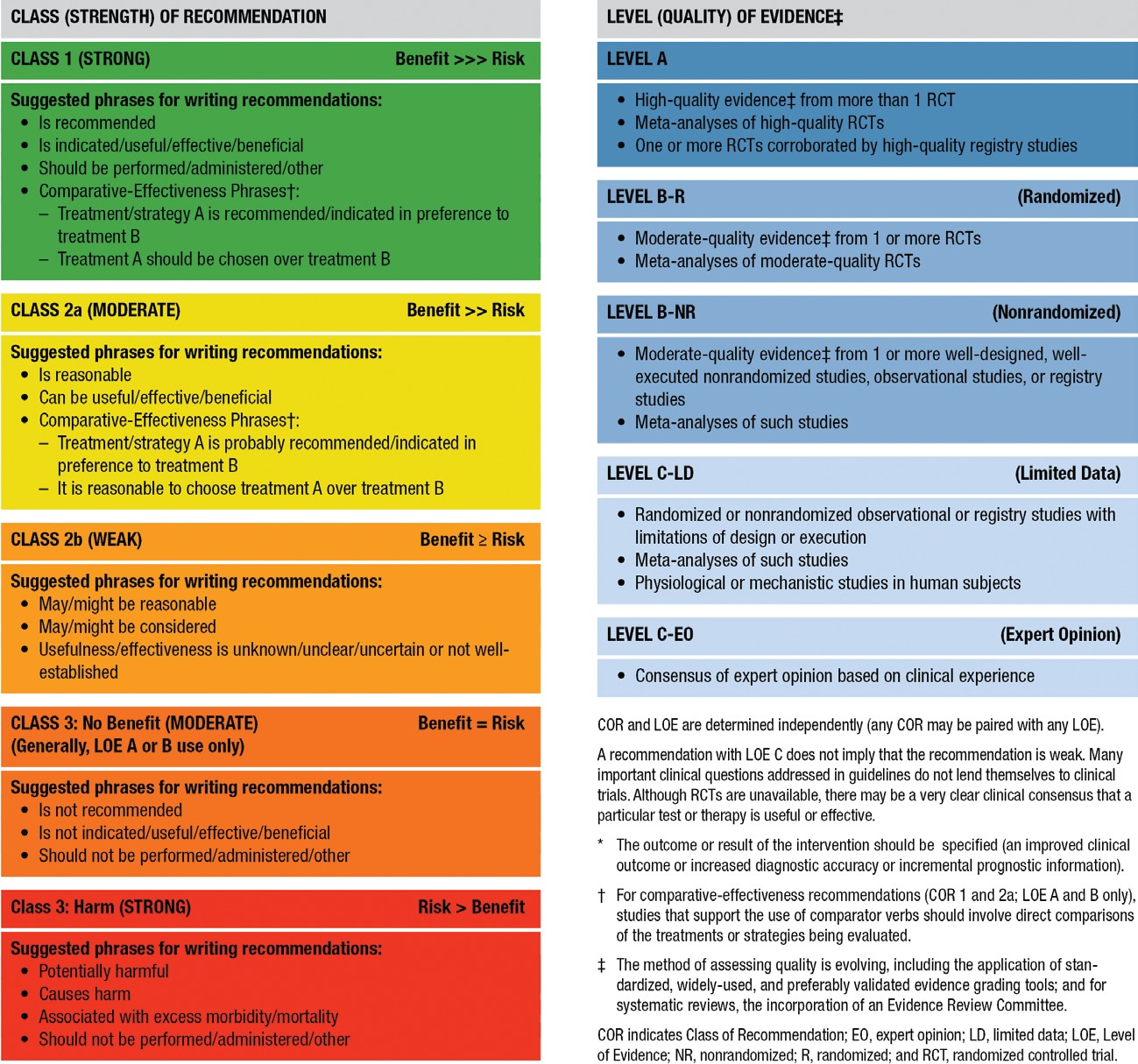
Guideline Structure
These guidelines are organized into knowledge chunks, grouped into discrete modules of information on specific topics or management issues.9,10 Each modular knowledge chunk includes a table of recommendations that uses the standard AHA nomenclature of COR and LOE. Recommendations are presented in order of COR: most potential benefit (Class 1), followed by lesser certainty of benefit (Class 2), and finally potential for harm or no benefit (Class 3). Following the COR, recommendations are ordered by the certainty of supporting LOE: Level A (high-quality randomized controlled trials) to Level C-EO (expert opinion). A brief introduction puts the recommendations into context with important background information and overarching management or treatment concepts. Recommendation-specific text clarifies the rationale and key study data supporting the recommendations. Hyperlinked references facilitate quick access and review. All writing group members reviewed and approved the final manuscript.
©2023 American Academy of Pediatrics and American Heart Association Inc
Document Review and Approval
These guidelines were submitted for blinded peer review to 11 subject-matter experts nominated by the AHA and the AAP. Before appointment, all peer reviewers were required to disclose relationships with industry and any other conflicts of interest, and all disclosures were reviewed by AHA staff. Peer reviewer feedback was provided for guidelines in draft format and again in final format. All guidelines were reviewed and approved for publication by the AHA Science Advisory and Coordinating Committee, the AHA Executive Committee, and the AAP Executive Board. Comprehensive disclosure information for peer reviewers is listed in Appendix 2.
These recommendations augment the last full set of AHA recommendations for neonatal resuscitation made in 2020.7 All other recommendations and algorithms published in the 2020 guidelines remain the official recommendations of the AHA Emergency Cardiovascular Care Science Subcommittee and writing groups.
REFERENCES
-
- Gomersall J, Berber S, Middleton P, McDonald SJ, Niermeyer S, El-Naggar W, Davis PG, Schmolzer GM, Ovelman C, Soll RF, et al. Umbilical cord management at term and late preterm birth: a meta-analysis. Pediatrics. 2021;147:e2020015404. doi: 10.1542/peds.2020-015404
- Seidler AL, Gyte GML, Rabe H, Diaz-Rossello JL, Duley L, Aziz K, Testoni Costa-Nobre D, Davis PG, Schmolzer GM, Ovelman C, et al. Umbilical cord management for newborns <34 weeks’ gestation: a meta-analysis. Pediatrics. 2021;147:e20200576. doi: 10.1542/peds.2020-0576
- Trevisanuto D, Roehr CC, Davis PG, Schmölzer GM, Wyckoff MH, Liley HG, Rabi Y, Weiner GM. Devices for administering ventilation at birth: a systematic review. Pediatrics. 2021;148:e2021050174. doi: 10.1542/peds.2021-050174
- Yamada NK, McKinlay CJ, Quek BH, Schmölzer GM, Wyckoff MH, Liley HG, Rabi Y, Weiner GM. Supraglottic airways compared with face masks for neonatal resuscitation: a systematic review. Pediatrics. 2022;150:e2022056568. doi: 10.1542/peds.2022-056568
- Wyckoff MH, Singletary EM, Soar J, Olasveengen TM, Greif R, Liley HG, Zideman D, Bhanji F, Andersen LW, Avis SR, et al. 2021 International consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations: summary from the Basic Life Support; Advanced Life Support; Neonatal Life Support; Education, Implementation, and Teams; First Aid Task Forces; and the COVID-19 Working Group. Circulation. 2022;145:e645–e721. doi: 10.1161/CIR.0000000000001017
- Wyckoff MH, Greif R, Morley PT, Ng K-C, Olasveengen TM, Singletary EM, Soar J, Cheng A, Drennan IR, Liley HG, et al. 2022 International consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations: summary from the Basic Life Support; Advanced Life Support; Pediatric Life Support; Neonatal Life Support; Education, Implementation, and Teams; and First Aid Task Forces. Circulation. 2022;146:e483–e557. doi: 10.1161/CIR.0000000000001095
- Aziz K, Lee HC, Escobedo MB, Hoover AV, Kamath-Rayne BD, Kapadia VS, Magid DJ, Niermeyer S, Schmolzer GM, Szyld E, et al. Part 5: neonatal resuscitation: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2020;142(16_suppl_2):S524–S550. doi: 10.1161/CIR.0000000000000902
- Wyckoff MH, Wyllie J, Aziz K, de Almeida MF, Fabres J, Fawke J, Guinsburg R, Hosono S, Isayama T, Kapadia VS, et al. Neonatal life support: 2020 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2020;142:S185–S221. doi: 10.1161/CIR.0000000000000895
- Magid DJ, Aziz K, Cheng A, Hazinski MF, Hoover AV, Mahgoub M, Panchal AR, Sasson C, Topjian AA, Rodriguez AJ, et al. Part 2: evidence evaluation and guidelines development: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2020;142:S358–S365. doi: 10.1161/CIR.0000000000000898
- Levine GN, O’Gara PT, Beckman JA, Al-Khatib SM, Birtcher KK, Cigarroa JE, de Las Fuentes L, Deswal A, Fleisher LA, Gentile F, et al. Recent innovations, modifications, and evolution of ACC/AHA clinical practice guidelines: an update for our constituencies: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e879–e886. doi: 10.1161/cir.0000000000000651
©2023 American Academy of Pediatrics and American Heart Association Inc
UMBILICAL CORD MANAGEMENT
Background
Management of the umbilical cord and placental transfusion at delivery remains an area of robust investigation. The volume of blood transferred from the placenta to the newborn infant and the effect of that transfusion vary on the basis of gestational age at delivery, mode of delivery, the time from delivery to cord clamping, any milking of the umbilical cord, and physiological status of the newborn infant.
Both the American College of Obstetrics and Gynecology and the AAP have produced guidelines about umbilical cord management.1,2 The most recent guidance from the AHA was published in 2020 and included all gestational ages and methods of placental transfusion.3 However, the developmental differences between preterm, late preterm, and term infants affect the outcomes of different cord management strategies, including the need for cardiorespiratory support in the delivery room, rate of moderate-to-severe hypoxic-ischemic encephalopathy, hematologic indices, need for admission to the neonatal intensive care unit, and use of therapeutic hypothermia. Because of these variations, ILCOR subsequently performed 2 different systematic reviews for cord management, 1 for late preterm and term infants4 and 1 for preterm infants.5 This focused update provides separate recommendations for these 2 distinct groups and relevant evidence published since those meta-analyses through September 2022.
Recommendations for Term/Late Preterm Newborn Umbilical Cord Management COR LOE Recommendations 2a B-R 1. For term and late preterm newborn infants ≥34 weeks’ gestation who do not require resuscitation, delayed cord clamping (DCC) (≥30 seconds) can be beneficial when compared to early cord clamping (<30 seconds). 2b B-R 2. For nonvigorous term and late preterm infants (35–42 weeks’ gestation), intact cord milking may be reasonable when compared to early cord clamping (<30 seconds). 3: No Benef it
C-LD 3. For term and late preterm newborn infants ≥34 weeks’ gestation who do not require resuscitation, intact cord milking is not known to be beneficial when compared to DCC (≥30 seconds). Recommendation-Specific Supportive Text
-
- Four RCTs (537 infants) found no difference in mortality between early and late cord clamping for term and late preterm infants.6–9 Low-certainty evidence from 15 studies (2641 infants) shows that DCC results in increased early hematologic indices (either hemoglobin or hematocrit) compared with early cord clamping.7,8,10–22
- One RCT including 1730 nonvigorous newborn infants limited to 35 to 42 weeks’ gestation (not including infants 34
©2023 American Academy of Pediatrics and American Heart Association Inc
weeks’ gestation) comparing intact umbilical cord milking with early cord clamping found no difference in the primary outcome of admission to the neonatal intensive care unit. However, differences in several secondary outcomes (including increased hemoglobin levels and a reduced need for cardiorespiratory support) make umbilical cord milking in this population a reasonable option.23 Additional studies would be helpful in further evaluating this intervention.
-
- There is no evidence available to support intact cord milking compared with DCC in vigorous term and late preterm infants.
Recommendations for Preterm Newborn Umbilical Cord Management COR LOE Recommendations 2a B-R 1. For preterm newborn infants <34 weeks’ gestation who do not require resuscitation, delaying cord clamping (≥30 seconds) can be beneficial when compared to early cord clamping (<30 seconds). 2b B-R 2. For preterm newborn infants between 28 and 34 weeks’ gestation who do not require resuscitation and in whom DCC cannot be performed, intact cord milking may be reasonable. 3: No Benef it
B-R 3. For preterm newborn infants <28 weeks’ gestation, intact cord milking is not recommended. Recommendation-Specific Supportive Text
- Sixteen RCTs (2988 infants) showed possible improvement in survival to discharge for infants receiving DCC compared with early cord clamping. DCC varied from 30 seconds to >2 minutes.24–39 Six studies (351 infants) showed that infants receiving DCC had decreased inotrope use in the first 24 hours.33,36–38,40,41 Infants receiving DCC had improved hematologic indices within 24 hours and 7 days,24–27,31–43 and received fewer red blood cell transfusions during admission.24,27,28,32,35,37–40,42–44
- Intact cord milking a.
Versus early cord clamping: In 11 trials (983 infants), infants receiving intact cord milking had higher hematologic indices in the first 24 hours.43,45–55 In 5 trials (439 infants), infants receiving intact cord milking received fewer inotropes in the first 24 hours.45–47,49,55 In infants 28 to 32 weeks’ gestation, 10 studies (889 infants) could not exclude benefit or harm of intact cord milking for severe intraventricular hemorrhage.43,45–47,49,51–53,55,56
b.
Versus DCC: A single trial including 292 preterm infants (28–31+6/7 weeks’ gestation) showed no increased risk of
severe intraventricular hemorrhage for umbilical cord milking compared with DCC.57
- In a single study of 182 infants born 23 to 27+6/7 weeks’ gestation not requiring resuscitation, severe intraventricular hemorrhage was significantly higher in those who received umbilical cord milking compared with DCC.57
©2023 American Academy of Pediatrics and American Heart Association Inc
REFERENCES
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice. Delayed umbilical cord clamping after
birth: ACOG committee opinion, Number 814. Obstet Gynecol. 2020;136:e100–e106. doi: 10.1097/AOG.0000000000004167
- American Academy of Pediatrics. Delayed umbilical cord clamping after birth. Pediatrics. 2017;139:e2017095. doi: 10.1542/peds.2017-0957
- Aziz K, Lee HC, Escobedo MB, Hoover AV, Kamath-Rayne BD, Kapadia VS, Magid DJ, Niermeyer S, Schmolzer GM, Szyld E, et al. Part 5: neonatal resuscitation: 2020 American Heart Association Guidelines for Cardiopulmonary -Resuscitation and Emergency -Cardiovascular Care. Circulation. 2020;142(16_suppl_2):S524–S550. doi: 10.1161/CIR.0000000000000902
- Gomersall J, Berber S, Middleton P, McDonald SJ, Niermeyer S, El-Naggar W, Davis PG, Schmolzer GM, Ovelman C, Soll RF, et al. Umbilical cord management at term and late preterm birth: a meta-analysis. Pediatrics. 2021;147:e2020015404. doi: 10.1542/peds.2020-015404
- Seidler AL, Gyte GML, Rabe H, Diaz-Rossello JL, Duley L, Aziz K, Testoni Costa-Nobre D, Davis PG, Schmolzer GM, Ovelman C, et al. Umbilical cord management for newborns <34 weeks’ gestation: a meta-analysis. Pediatrics. 2021;147:e20200576. doi: 10.1542/peds.2020-0576
- Backes CH, Huang H, Cua CL, Garg V, Smith CV, Yin H, Galantowicz M, Bauer JA, Hoffman TM. Early versus delayed umbilical cord clamping in infants with congenital heart disease: a pilot, randomized, controlled trial. J Perinatol. 2015;35:826–831. doi: 10.1038/jp.2015.89
- Ceriani Cernadas JM, Carroli G, Pellegrini L, Otano L, Ferreira M, Ricci C, Casas O, Giordano D, Lardizabal J. The effect of timing of cord clamping on neonatal venous hematocrit values and clinical outcome at term: a randomized, controlled trial. Pediatrics. 2006;117:e779–e786. doi: 10.1542/peds.2005-1156
- Chopra A, Thakur A, Garg P, Kler N, Gujral K. Early versus delayed cord clamping in small for gestational age infants and iron stores at 3 months of age: a randomized controlled trial. BMC Pediatr. 2018;18:234. doi: 10.1186/s12887-018-1214-8
- Datta BV, Kumar A, Yadav R. A randomized controlled trial to evaluate the role of brief delay in cord clamping in preterm neonates (34–36 weeks) on short-term neurobehavioural outcome. J Trop Pediatr. 2017;63:418–424. doi: 10.1093/tropej/fmx004
- Al-Tawil MM, Abdel-Aal MR, Kaddah MA. A randomized controlled trial on delayed cord clamping and iron status at 3–5 months in term neonates held at the level of maternal pelvis. J Neonatal-Perinat Med. 2012;5:319–326. doi: 10.3233/npm-1263112
- Chaparro CM, Neufeld LM, Tena Alavez G, Eguia-Liz Cedillo R, Dewey KG. Effect of timing of umbilical cord clamping on iron status in Mexican infants: a randomised controlled trial. Lancet. 2006;367:1997–2004. doi: 10.1016/S0140-6736(06)68889-2
- Chen X, Li X, Chang Y, Li W, Cui H. Effect and safety of timing of cord clamping on neonatal hematocrit values and clinical outcomes in term infants: a randomized controlled trial. J Perinatol. 2018;38:251–257. doi: 10.1038/s41372-017-0001-y
- De Paco C, Herrera J, Garcia C, Corbalán S, Arteaga A, Pertegal M, Checa R, Prieto MT, Nieto A, Delgado JL. Effects of delayed cord clamping on the third stage of labour, maternal haematological parameters and acid-base status in fetuses at term. Eur J Obstet Gynecol Reprod Biol. 2016;207:153–156. doi: 10.1016/j.ejogrb.2016.10.031
- Emhamed MO, van Rheenen P, Brabin BJ. The early effects of delayed cord clamping in term infants born to Libyan mothers.
Trop Doct. 2004;34:218–222. doi: 10.1177/004947550403400410
- Fawzy AE-MA, Moustafa , El-Kassar AA, El-Kassar YS, Swelem MS, El-Agwany AS, Diab DA. Early versus delayed cord clamping of term births in Shatby Maternity University Hospital. Prog Obstet Ginecol. 2015;58:389–392. doi: 10.1016/j.pog.2015.05.001
- Jahazi A, Kordi M, Mirbehbahani NB, Mazloom SR. The effect of early and late umbilical cord clamping on neonatal hematocrit.
J Perinatol. 2008;28:523–525. doi: 10.1038/jp.2008.55
- Mohammad K, Tailakh S, Fram K, Creedy D. Effects of early umbilical cord clamping versus delayed clamping on maternal and neonatal outcomes: a Jordanian study. J Matern Fetal Neonatal Med. 2021;34:231–237. doi: 10.1080/14767058.2019.1602603
- Philip AG. Further observations on placental transfusion. Obstet Gynecol. 1973;42:334–343.
- Salari Z, Rezapour M, Khalili N. Late umbilical cord clamping, neonatal hematocrit and Apgar scores: a randomized controlled trial. J Neonatal Perinatal Med. 2014;7:287–291. doi: 10.3233/NPM-1463913
- Ultee CA, van der Deure J, Swart J, Lasham C, van Baar AL. Delayed cord clamping in preterm infants delivered at 34–36 weeks’
gestation: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2008;93:F20–F23. doi: 10.1136/adc.2006.100354
- Vural I, Ozdemir H, Teker G, Yoldemir T, Bilgen H, Ozek E. Delayed cord clamping in term large-for-gestational age infants: a prospective randomised study. J Paediatr Child Health. 2019;55:555–560. doi: 10.1111/jpc.14242
- Yadav AK, Upadhyay A, Gothwal S, Dubey K, Mandal U, Yadav CP. Comparison of three types of intervention to enhance placental redistribution in term newborns: randomized control trial. J Perinatol. 2015;35:720–724. doi: 10.1038/jp.2015.65
- Katheria AC, Clark E, Yoder B, Schmolzer GM, Yan Law BH, El-Naggar W, Rittenberg D, Sheth S, Mohamed MA, Martin C, et al. Umbilical cord milking in nonvigorous infants: a cluster-randomized crossover trial. Am J Obstet Gynecol. 2023;228:217.e1–217.e14. doi: 10.1016/j.ajog.2022.08.015
©2023 American Academy of Pediatrics and American Heart Association Inc
- Armanian AM, Tehrani HG, Ansari M, Ghaemi S. Is “delayed umbilical cord clamping” beneficial for premature newborns?. Int J Pediatr. 2017;5:4909–4918. doi: 10.22038/ijp.2016.7909
- Backes CH, Huang H, Iams JD, Bauer JA, Giannone PJ. Timing of umbilical cord clamping among infants born at 22 through 27
weeks’ gestation. J Perinatol. 2016;36:35–40. doi: 10.1038/jp.2015.117
- Baenziger O, Stolkin F, Keel M, von Siebenthal K, Fauchere JC, Das Kundu S, Dietz V, Bucher HU, Wolf M. The influence of the timing of cord clamping on postnatal cerebral oxygenation in preterm neonates: a randomized, controlled trial. Pediatrics. 2007;119:455–459. doi: 10.1542/peds.2006-2725
- Das B, Sundaram V, Kumar P, Mordi WT, Dhaliwal LK, Das R. Effect of placental transfusion on iron stores in moderately preterm neonates of 30–33 weeks gestation. Indian J Pediatr. 2018;85:172–178. doi: 10.1007/s12098-017-2490-2
- Duley L, Dorling J, Pushpa-Rajah A, Oddie SJ, Yoxall CW, Schoonakker B, Bradshaw L, Mitchell EJ, Fawke JA, Cord Pilot Trial Collaborative Group. Randomised trial of cord clamping and initial stabilisation at very preterm birth. Arch Dis Child Fetal Neonatal Ed. 2018;103:F6–F14. doi: 10.1136/archdischild-2016-312567
- Hofmeyr GJ, Bolton KD, Bowen DC, Govan JJ. Periventricular/intraventricular haemorrhage and umbilical cord clamping. Findings and hypothesis. S Afr Med J. 1988;73:104–106.
- Hofmeyr GJ, Gobetz L, Bex PJ, Van der Griendt M, Nikodem C, Skapinker R, Delahunt T. Periventricular/intraventricular hemorrhage following early and delayed umbilical cord clamping. A randomized controlled trial. Online J Curr Clin Trials. 1993;Doc No 110.
- Kinmond S, Aitchison TC, Holland BM, Jones JG, Turner TL, Wardrop CA. Umbilical cord clamping and preterm infants: a randomised trial. BMJ. 1993;306:172–175. doi: 10.1136/bmj.306.6871.172
- Kugelman A, Borenstein-Levin L, Riskin A, Chistyakov I, Ohel G, Gonen R, Bader D. Immediate versus delayed umbilical cord clamping in premature neonates born <35 weeks: a prospective, randomized, controlled study. Am J Perinatol. 2007;24:307–315. doi: 10.1055/s-2007-981434
- McDonnell M, Henderson-Smart DJ. Delayed umbilical cord clamping in preterm infants: a feasibility study. J Paediatr Child Health. 1997;33:308–310. doi: 10.1111/j.1440-1754.1997.tb01606.x
- Mercer JS, McGrath MM, Hensman A, Silver H, Oh W. Immediate and delayed cord clamping in infants born between 24 and 32 weeks: a pilot randomized controlled trial. J Perinatol. 2003;23:466–472. doi: 10.1038/sj.jp.7210970
- Mercer JS, Vohr BR, Erickson-Owens DA, Padbury JF, Oh W. Seven-month developmental outcomes of very low birth weight infants enrolled in a randomized controlled trial of delayed versus immediate cord clamping. J Perinatol. 2010;30:11–16. doi: 10.1038/jp.2009.170
- Oh W, Fanaroff AA, Carlo WA, Donovan EF, McDonald SA, Poole WK. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Effects of delayed cord clamping in very-low-birth-weight infants. J Perinatol. 2011;31(suppl 1):S68–S71. doi: 10.1038/jp.2010.186
- Rabe H, Wacker A, Hulskamp G, Hornig-Franz I, Schulze-Everding A, Harms E, Cirkel U, Louwen F, Witteler R, Schneider HP. A randomised controlled trial of delayed cord clamping in very low birth weight preterm infants. Eur J Pediatr. 2000;159:775–777. doi: 10.1007/pl00008345
- Ruangkit C, Bumrungphuet S, Panburana P, Khositseth A, Nuntnarumit P. A randomized controlled trial of immediate versus delayed umbilical cord clamping in multiple-birth infants born preterm. Neonatology. 2019;115:156–163. doi: 10.1159/000494132
- Tarnow-Mordi W, Morris J, Kirby A, Robledo K, Askie L, Brown R, Evans N, Finlayson S, Fogarty M, Gebski V, et al. Delayed versus immediate cord clamping in preterm infants. N Engl J Med. 2017;377:2445–2455. doi: 10.1056/NEJMoa1711281
- Dong XY, Sun XF, Li MM, Yu ZB, Han SP. Influence of delayed cord clamping on preterm infants with a gestational age of <32
weeks [in Chinese]. Zhongguo Dang Dai Er Ke Za Zhi. 2016;18:635–638. doi: 10.7499/j.issn.1008-8830.2016.07.013
- Gokmen Z, Ozkiraz S, Tarcan A, Kozanoglu I, Ozcimen EE, Ozbek N. Effects of delayed umbilical cord clamping on peripheral blood hematopoietic stem cells in premature neonates. J Perinat Med. 2011;39:323–329. doi: 10.1515/jpm.2011.021
- Dipak NK, Nanavat RN, Kabra NK, Srinivasan A, Ananthan A. Effect of delayed cord clamping on hematocrit, and thermal and hemodynamic stability in preterm neonates: a randomized controlled trial. Indian Pediatr. 2017;54:112–115. doi: 10.1007/s13312-017-1011-8
- Finn D, Ryan DH, Pavel A, O’Toole JM, Livingstone V, Boylan GB, Kenny LC, Dempsey EM. Clamping the umbilical cord in premature deliveries
(CUPiD): neuromonitoring in the immediate newborn period in a randomized, controlled trial of preterm infants born at <32
weeks of gestation. J Pediatr. 2019;208:121–126.e122. doi: 10.1016/j.jpeds.2018.12.039
- Rana A, Agarwal K, Ramji S, Gandhi G, Sahu L. Safety of delayed umbilical cord clamping in preterm neonates of less than 34 weeks of gestation: a randomized controlled trial. Obstet Gynecol Sci. 2018;61:655–661. doi: 10.5468/ogs.2018.61.6.655
- Elimian A, Goodman J, Escobedo M, Nightingale L, Knudtson E, Williams M. Immediate compared with delayed cord clamping in the preterm neonate: a randomized controlled trial. Obstet Gynecol. 2014;124:1075–1079. doi: 10.1097/AOG.0000000000000556
- El-Naggar W, Simpson D, Hussain A, Armson A, Dodds L, Warren A, Whyte R, McMillan D. Cord milking versus immediate clamping in preterm infants: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2019;104:F145–F150. doi: 10.1136/archdischild-2018-314757
©2023 American Academy of Pediatrics and American Heart Association Inc
- Hosono S, Mugishima H, Fujita H, Hosono A, Minato M, Okada T, Takahashi S, Harada K. Umbilical cord milking reduces the need for red cell transfusions and improves neonatal adaptation in infants born at less than 29 weeks’ gestation: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2008;93:F14–F19. doi: 10.1136/adc.2006.108902
- Katheria A, Blank D, Rich W, Finer N. Umbilical cord milking improves transition in premature infants at birth. PLoS One. 2014;9:e94085. doi: 10.1371/journal.pone.0094085
- Katheria AC, Leone TA, Woelkers D, Garey DM, Rich W, Finer NN. The effects of umbilical cord milking on hemodynamics and neonatal outcomes in premature neonates. J Pediatr. 2014;164:1045–1050.e1. doi: 10.1016/j.jpeds.2014.01.024
- Kilicdag H, Gulcan H, Hanta D, Torer B, Gokmen Z, Ozdemir SI, Antmen BA. Is umbilical cord milking always an advantage? J Matern Fetal Neonatal Med. 2016;29:615–618. doi: 10.3109/14767058.2015.1012067
- Li J, Yu B, Wang W, Luo D, Dai QL, Gan XQ. Does intact umbilical cord milking increase infection rates in preterm infants with premature prolonged rupture of membranes?. J Matern Fetal Neonatal Med. 2020;33:184–190. doi: 10.1080/14767058.2018.1487947
- March MI, Hacker MR, Parson AW, Modest AM, de Veciana M. The effects of umbilical cord milking in extremely preterm infants: a randomized controlled trial. J Perinatol. 2013;33:763–767. doi: 10.1038/jp.2013.70
- Mercer JS, Erickson-Owens DA, Vohr BR, Tucker RJ, Parker AB, Oh W, Padbury JF. Effects of placental transfusion on neonatal and 18 month outcomes in preterm infants: a randomized controlled trial. J Pediatr. 2016;168:50–55.e51. doi: 10.1016/j.jpeds.2015.09.068
- Silahli M, Duman E, Gokmen Z, Toprak E, Gokdemir M, Ecevit A. The relationship between placental transfusion, and thymic size and neonatal morbidities in premature infants: a randomized control trial. J Pakistan Med Assoc. 2018;68:1560–1565.
- Song SY, Kim Y, Kang BH, Yoo HJ, Lee M. Safety of umbilical cord milking in very preterm neonates: a randomized controlled study. Obstet Gynecol Sci. 2017;60:527–534. doi: 10.5468/ogs.2017.60.6.527
- Alan S, Arsan S, Okulu E, Akin IM, Kilic A, Taskin S, Cetinkaya E, Erdeve O, Atasay B. Effects of umbilical cord milking on the need for packed red blood cell transfusions and early neonatal hemodynamic adaptation in preterm infants born ≤1500 g: a prospective, randomized, controlled trial. J Pediatr Hematol Oncol. 2014;36:e493–e498. doi: 10.1097/MPH.0000000000000143
- Katheria A, Reister F, Essers J, Mendler M, Hummler H, Subramaniam A, Carlo W, Tita A, Truong G, Davis-Nelson S, et al. Association of umbilical cord milking vs delayed umbilical cord clamping with death or severe intraventricular hemorrhage among preterm infants. JAMA. 2019;322:1877–1886. doi: 10.1001/jama.2019.16004
VENTILATORY SUPPORT AFTER BIRTH: DEVICES AND INTERFACES TO ADMINISTER PPV
Background
The 2020 AHA guidelines provided recommendations for when and how to provide PPV, including guidance for
-inflation pressures, the use of positive end-expiratory pressure, ventilation rate, and inspiratory time.1 The recommendations provided in this focused update discuss devices to deliver PPV, and the choice between a face mask and a supraglottic airway as the interface used for PPV.
Several devices are available to administer PPV, including self-inflating bags, flow-inflating bags, and T-piece resuscitators. The choice of PPV device depends on factors reflecting the context at a birthing site: the number of births, the case mix, availability of a compressed gas source, familiarity with the different devices, amount of training required to use each device, and device cost. Because both T-piece resuscitators and flow-inflating bags require a compressed gas source to function, a self-inflating bag should be available as a backup in the event of compressed gas failure when using either of these devices.
Available interfaces for PPV delivery include face masks, nasal prongs, and supraglottic airways. This focused update specifically addresses the choice between face masks and supraglottic airways as the primary interface during PPV.
©2023 American Academy of Pediatrics and American Heart Association Inc
Recommendations for Devices Used to Administer PPV for Newborn Infants COR LOE Recommendations 2a B-NR 1. It can be beneficial to use a T-piece resuscitator instead of a self-inflating bag, with or without a positive end-expiratory pressure valve, for administering positive-pressure ventilation to newborn infants, particularly for preterm infants.
Recommendation-Specific Supportive Text
-
- A meta-analysis of 4 RCTs (1247 term and preterm infants) found that resuscitation with a T-piece resuscitator compared with a self-inflating bag reduced the duration of PPV and decreased risk of bronchopulmonary dysplasia.2 Although subgroup analyses by gestational age were not feasible in this meta-analysis, bronchopulmonary dysplasia is an outcome that affects preterm infants, and the use of a T-piece resuscitator may present the greatest benefit to preterm infants. The systematic review did not identify any studies that evaluated the use of flow-inflating bags.2
Recommendation for Interfaces Used to Administer PPV for Newborn Infants COR LOE Recommendation 2b C-LD 1. It may be reasonable to use a supraglottic airway as the primary interface to administer PPV instead of a face mask for newborn infants delivered at ≥34 0/7 weeks’ gestation.
Recommendation-Specific Supportive Text
1. A meta-analysis of 6 RCTs (1823 infants delivered at ≥34 0/7 weeks’ gestation) found that use of a supraglottic airway decreased the probability of failure to improve with the assigned device, and the rate of endotracheal intubation in the delivery room.3 Failure to improve with the assigned device was a pragmatic outcome chosen to assess whether primary use of the supraglottic airway or face mask to provide PPV led to improvement of neonates undergoing resuscitation after birth. The duration of PPV and time until heart rate reached >100/min were also shorter with the supraglottic airway.
©2023 American Academy of Pediatrics and American Heart Association Inc
Based on available evidence, this recommendation is limited to newborn infants ≥34 0/7 weeks’ gestation. All studies included in this meta-analysis were performed in lower-resourced settings. No studies have compared face masks with supraglottic devices for initiating PPV during neonatal resuscitation in high-resourced settings. As a result, the effect size reported in this meta-analysis may not be generalizable to settings with greater availability of health care practitioners with advanced skills and highly trained neonatal resuscitation teams.
REFERENCES
- Aziz K, Lee HC, Escobedo MB, Hoover AV, Kamath-Rayne BD, Kapadia VS, Magid DJ, Niermeyer S, Schmolzer GM, Szyld E, et al. Part 5: neonatal resuscitation: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2020;142(16_suppl_2):S524–S550. doi: 10.1161/CIR.0000000000000902
- Trevisanuto D, Roehr CC, Davis PG, Schmölzer GM, Wyckoff MH, Liley HG, Rabi Y, Weiner GM. Devices for administering ventilation at birth: a systematic review. Pediatrics. 2021;148:e2021050174, doi: 10.1542/peds.2021-050174
- Yamada NK, McKinlay CJ, Quek BH, Schmölzer GM, Wyckoff MH, Liley HG, Rabi Y, Weiner GM. Supraglottic airways compared with face masks for neonatal resuscitation: a systematic review. Pediatrics. 2022;150:e2022056568. doi: 10.1542/peds.2022-056568
KNOWLEDGE GAPS AND PRIORITIES OF RESEARCH ACKNOWLEDGMENTS
This focused update is limited to a review of the science on umbilical cord management in term, late preterm, and preterm newborn infants and on the devices and interfaces used for administering PPV during resuscitation of newborn infants. The topics in the knowledge chunks of this update contain additional questions and practices for which evidence was weak, uncertain, or absent. In addition, the following knowledge gaps require further research:
Umbilical Cord Management
-
- Optimal management of the umbilical cord in term, late preterm, and preterm infants who require resuscitation at
delivery
-
- Longer-term outcome data, such as anemia during infancy and neurodevelopmental outcomes, for all umbilical cord management strategies
Devices for Administering PPV
-
- Cost-effectiveness of a T-piece resuscitator compared with a self-inflating bag
- The effect of a self-inflating bag with a positive end-expiratory pressure valve on outcomes in preterm newborn infants
- Comparison of either a T-piece resuscitator or a self-inflating bag with a flow-inflating bag for administering PPV
- Comparison of clinical outcomes by gestational age for any PPV device
Interfaces for Administering PPV
-
- Comparison of supraglottic airway devices and face masks as the primary interface for PPV in high-resourced settings
- The amount and type of training required for successful supraglottic airway insertion and the potential for skill decay
- The utility of supraglottic airway devices for suctioning secretions from the airway
- The efficacy of a supraglottic airway during advanced neonatal resuscitation requiring chest compressions or the
delivery of intratracheal medications
©2023 American Academy of Pediatrics and American Heart Association Inc
ARTICLE INFORMATION
The American Heart Association and the American Academy of Pediatrics make every effort to avoid any actual or potential conflicts of interest that may arise as a result of an outside relationship or a personal, professional, or business interest of a member of the writing panel. Specifically, all members of the writing group are required to complete and submit a Disclosure Questionnaire showing all such relationships that might be perceived as real or potential conflicts of interest.
This focused update was approved by the American Heart Association Science Advisory and Coordinating Committee on July 15, 2023; the American Heart Association Executive Committee on August 4, 2023; and the American Academy of Pediatrics on July 6, 2023.
This article has been copublished in Circulation.
The expert peer review of AHA-commissioned documents (eg, scientific statements, clinical practice guidelines, systematic reviews) is conducted by the AHA Office of Science Operations. For more on AHA statements and guidelines development, visit https://professional.heart.org/statements. Select the “Guidelines & Statements” drop-down menu, then click “Publication Development.”
Disclosures
Appendix 1. Writing Group Disclosures
Writing group member Employment Research grant Other research support Speakers’ bureau/honora ria Expert witness Ownership interest Consultant/ advisory board Other Henry C. Lee University of California San Diego None None None None None None None Edgardo Szyld Indiana University None None None None None None None Emer Finan Mount Sinai Hospital; University of Toronto None None None None None None None Jessica L. Illuzzi Yale School of Medicine None None None None None None None Beena D. Kamath-Rayne American Academy of Pediatrics None None None None None None Cerebral Palsy Alliance Research Foundation* Vishal S. Kapadia UT Southwestern NIH† Masimo Corporation†
None None None None None None Susan Niermeyer University of Colorado NIH* None None None None None None Georg M. Schmölzer University of Alberta CIHR Grant† CIHR Grant† None None None None None None Marya L. Strand Akron Children’s Hospital
None None None None None None None Gary M. Weiner University of Michigan None None None None None None None Amanda Williams Cedars Sinai None None None None None None None Myra H. Wyckoff UT Southwestern None None None None None None None Nicole K. Yamada Stanford University AHRQ†; AHRQ* None None None None None None This table represents the relationships of writing group members that may be perceived as actual or reasonably perceived conflicts of interest as reported on the Disclosure Questionnaire, which all members of the writing group are required to complete and submit. A relationship is considered to be “significant” if (a) the person receives $5000 or more during any 12-month period, or 5% or more of the person’s gross income; or (b) the person owns 5% or more of the voting stock or share of the entity, or owns $5000 or more of the fair market value of the entity. A relationship is considered to be “modest” if it is less than “significant” under the preceding definition.
*Modest.
†Significant.
©2023 American Academy of Pediatrics and American Heart Association Inc
Appendix 2. Reviewer Disclosures
Reviewer Employment Research grant Other research support Speakers’ bureau/honora ria Expert witness Ownership interest Consultant/ advisory board Other Ryan Alanzalon Kaiser Permanente None None None None None None None Rakhi G. Basuray The Ohio State University, Nationwide Children’s Hospital None None None None None None None Justin Goldstein Beth Israel Deaconess Medical Center None None None None None None None Arun Gupta Lucile Packard Children’s Hospital at Stanford University None None None None None None None Lia Lowrie St. Louis University School of Medicine None None None None None None None Allison Markowsky Children’s National Hospital
None None None None None None None Saurabhkumar C. Patel
University of Illinois Chicago None None None None None None None Betsy Peterson SOAPM None None None None None None None Clara Song Kaiser Permanente None None None None None None None Michael R. Stenger The Ohio State -University
None None None None None None None Muhammad Waseem Lincoln Medical Center None None None None None None None This table represents the relationships of reviewers that may be perceived as actual or reasonably perceived conflicts of interest as reported on the Disclosure Questionnaire, which all reviewers are required to complete and submit. A relationship is considered to be “significant” if (a) the person receives $5000 or more during any 12-month period, or 5% or more of the person’s gross income; or (b) the person owns 5% or more of the voting stock or share of the entity, or owns $5000 or more of the fair market value of the entity. A relationship is considered to be “modest” if it is less than “significant” under the preceding definition.
©2023 American Academy of Pediatrics and American Heart Association Inc